Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition?
- PMID: 20943748
- PMCID: PMC3012196
- DOI: 10.2337/db10-0867
Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition?
Abstract
Objective: Lipotoxicity and ectopic fat deposition reduce insulin signaling. It is not clear whether excess fat deposition in nonadipose tissue arises from excessive fatty acid delivery from adipose tissue or from impaired adipose tissue storage of ingested fat.
Research design and methods: To investigate this we used a whole-body integrative physiological approach with multiple and simultaneous stable-isotope fatty acid tracers to assess delivery and transport of endogenous and exogenous fatty acid in adipose tissue over a diurnal cycle in lean (n = 9) and abdominally obese men (n = 10).
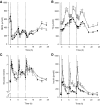
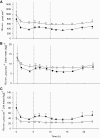
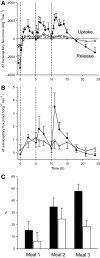
Results: Abdominally obese men had substantially (2.5-fold) greater adipose tissue mass than lean control subjects, but the rates of delivery of nonesterified fatty acids (NEFA) were downregulated, resulting in normal systemic NEFA concentrations over a 24-h period. However, adipose tissue fat storage after meals was substantially depressed in the obese men. This was especially so for chylomicron-derived fatty acids, representing the direct storage pathway for dietary fat. Adipose tissue from the obese men showed a transcriptional signature consistent with this impaired fat storage function.
Conclusions: Enlargement of adipose tissue mass leads to an appropriate downregulation of systemic NEFA delivery with maintained plasma NEFA concentrations. However the implicit reduction in adipose tissue fatty acid uptake goes beyond this and shows a maladaptive response with a severely impaired pathway for direct dietary fat storage. This adipose tissue response to obesity may provide the pathophysiological basis for ectopic fat deposition and lipotoxicity.
Figures

References
-
- Bjorntorp P: Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabete Metab Rev 1988;4:615–622 - PubMed
-
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS: INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–1649 - PubMed
-
- Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjønneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quirós JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E: General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–2120 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

