Cytosolic Ca2+ buffers
- PMID: 20943758
- PMCID: PMC2964180
- DOI: 10.1101/cshperspect.a004051
Cytosolic Ca2+ buffers
Abstract
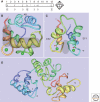
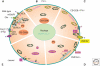
"Ca(2+) buffers," a class of cytosolic Ca(2+)-binding proteins, act as modulators of short-lived intracellular Ca(2+) signals; they affect both the temporal and spatial aspects of these transient increases in [Ca(2+)](i). Examples of Ca(2+) buffers include parvalbumins (α and β isoforms), calbindin-D9k, calbindin-D28k, and calretinin. Besides their proven Ca(2+) buffer function, some might additionally have Ca(2+) sensor functions. Ca(2+) buffers have to be viewed as one of the components implicated in the precise regulation of Ca(2+) signaling and Ca(2+) homeostasis. Each cell is equipped with proteins, including Ca(2+) channels, transporters, and pumps that, together with the Ca(2+) buffers, shape the intracellular Ca(2+) signals. All of these molecules are not only functionally coupled, but their expression is likely to be regulated in a Ca(2+)-dependent manner to maintain normal Ca(2+) signaling, even in the absence or malfunctioning of one of the components.
Figures
References
-
- Airaksinen L, Virkkala J, Aarnisalo A, Meyer M, Ylikoski J, Airaksinen MS 2000. Lack of calbindin-D28k does not affect hearing level or survival of hair cells in acoustic trauma. ORL J Otorhinolaryngol Relat Spec 62: 9–12 - PubMed
-
- Akke M, Forsen S, Chazin WJ 1991. Molecular basis for co-operativity in Ca2+ binding to calbindin D9k. 1H nuclear magnetic resonance studies of (Cd2+)1-bovine calbindin D9k. J Mol Biol 220: 173–189 - PubMed
-
- Allbritton NL, Meyer T, Stryer L 1992. Range of messenger action of calcium ion and inositol 1,4,5,-trisphophate. Science 258: 1812–1815 - PubMed
-
- Babini E, Bertini I, Capozzi F, Del Bianco C, Hollender D, Kiss T, Luchinat C, Quattrone A 2004. Solution structure of human beta-parvalbumin and structural comparison with its paralog alpha-parvalbumin and with their rat orthologs. Biochemistry 43: 16076–16085 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
