Gastrin-releasing peptide/neuromedin B receptor antagonists PD176252, PD168368, and related analogs are potent agonists of human formyl-peptide receptors
- PMID: 20943772
- PMCID: PMC3014281
- DOI: 10.1124/mol.110.068288
Gastrin-releasing peptide/neuromedin B receptor antagonists PD176252, PD168368, and related analogs are potent agonists of human formyl-peptide receptors
Abstract
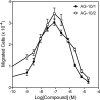
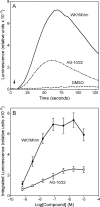
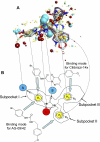
N-Formyl peptide receptors (FPRs) are G protein-coupled receptors (GPCRs) involved in host defense and sensing cellular dysfunction. Thus, FPRs represent important therapeutic targets. In the present studies, we screened 32 ligands (agonists and antagonists) of unrelated GPCRs for their ability to induce intracellular Ca²+ mobilization in human neutrophils and HL-60 cells transfected with human FPR1, FPR2, or FPR3. Screening of these compounds demonstrated that antagonists of gastrin-releasing peptide/neuromedin B receptors (BB₁/BB₂) PD168368 [(S)-a-methyl-a-[[[(4-nitrophenyl)amino]carbonyl]amino]-N-[[1-(2-pyridinyl) cyclohexyl]methyl]-1H-indole-3-propanamide] and PD176252 [(S)-N-[[1-(5-methoxy-2-pyridinyl)cyclohexyl]methyl]-a-methyl-a-[[-(4-nitrophenyl)amino]carbonyl]amino-1H-indole-3-propanamide] were potent mixed FPR1/FPR2 agonists, with nanomolar EC₅₀ values. Cholecystokinin-1 receptor agonist A-71623 [Boc-Trp-Lys(ε-N-2-methylphenylaminocarbonyl)-Asp-(N-methyl)-Phe-NH₂] was also a mixed FPR1/FPR2 agonist, but with a micromolar EC₅₀. Screening of 56 Trp- and Phe-based PD176252/PD168368 analogs and 41 related nonpeptide/nonpeptoid analogs revealed 22 additional FPR agonists. Most were potent mixed FPR1/FPR2/FPR3 agonists with nanomolar EC₅₀ values for FPR2, making them among the most potent nonpeptide FPR2 agonists reported to date. In addition, these agonists were also potent chemoattractants for murine and human neutrophils and activated reactive oxygen species production in human neutrophils. Molecular modeling of the selected agonists using field point methods allowed us to modify our previously reported pharmacophore model for the FPR2 ligand binding site. This model suggests the existence of three hydrophobic/aromatic subpockets and several binding poses of FPR2 agonists in the transmembrane region of this receptor. These studies demonstrate that FPR agonists could include ligands of unrelated GPCR and that analysis of such compounds can enhance our understanding of pharmacological effects of these ligands.
Figures


Similar articles
-
3-(1H-indol-3-yl)-2-[3-(4-nitrophenyl)ureido]propanamide enantiomers with human formyl-peptide receptor agonist activity: molecular modeling of chiral recognition by FPR2.Biochem Pharmacol. 2013 Feb 1;85(3):404-16. doi: 10.1016/j.bcp.2012.11.015. Epub 2012 Dec 3. Biochem Pharmacol. 2013. PMID: 23219934 Free PMC article.
-
Identification of novel small-molecule agonists for human formyl peptide receptors and pharmacophore models of their recognition.Mol Pharmacol. 2010 Feb;77(2):159-70. doi: 10.1124/mol.109.060673. Epub 2009 Nov 10. Mol Pharmacol. 2010. PMID: 19903830 Free PMC article.
-
Novel 3-(1H-indol-3-yl)-2-[3-(4-methoxyphenyl)ureido]propanamides as selective agonists of human formyl-peptide receptor 2.Bioorg Med Chem. 2015 Jul 15;23(14):3913-24. doi: 10.1016/j.bmc.2014.12.007. Epub 2014 Dec 13. Bioorg Med Chem. 2015. PMID: 25549897 Free PMC article.
-
Formyl peptide receptor 2 is an emerging modulator of inflammation in the liver.Exp Mol Med. 2023 Feb;55(2):325-332. doi: 10.1038/s12276-023-00941-1. Epub 2023 Feb 7. Exp Mol Med. 2023. PMID: 36750693 Free PMC article. Review.
-
International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family.Pharmacol Rev. 2009 Jun;61(2):119-61. doi: 10.1124/pr.109.001578. Epub 2009 Jun 4. Pharmacol Rev. 2009. PMID: 19498085 Free PMC article. Review.
Cited by
-
Machine learning algorithms for predicting glioma patient prognosis based on CD163+FPR3+ macrophage signature.NPJ Precis Oncol. 2024 Sep 13;8(1):201. doi: 10.1038/s41698-024-00692-w. NPJ Precis Oncol. 2024. PMID: 39271911 Free PMC article.
-
Therapeutic Potential of Polyphenols from Epilobium Angustifolium (Fireweed).Phytother Res. 2016 Aug;30(8):1287-97. doi: 10.1002/ptr.5648. Epub 2016 May 24. Phytother Res. 2016. PMID: 27215200 Free PMC article. Review.
-
Further studies on 2-arylacetamide pyridazin-3(2H)-ones: design, synthesis and evaluation of 4,6-disubstituted analogs as formyl peptide receptors (FPRs) agonists.Eur J Med Chem. 2013 Jun;64:512-28. doi: 10.1016/j.ejmech.2013.03.066. Epub 2013 Apr 8. Eur J Med Chem. 2013. PMID: 23685570 Free PMC article.
-
Annexin A1: potential for glucocorticoid sparing in RA.Nat Rev Rheumatol. 2013 Oct;9(10):595-603. doi: 10.1038/nrrheum.2013.126. Epub 2013 Aug 20. Nat Rev Rheumatol. 2013. PMID: 23958797 Review.
-
Molecular docking of 2-(benzimidazol-2-ylthio)-N-phenylacetamide-derived small-molecule agonists of human formyl peptide receptor 1.J Mol Model. 2012 Jun;18(6):2831-43. doi: 10.1007/s00894-011-1307-x. Epub 2011 Nov 30. J Mol Model. 2012. PMID: 22127612 Free PMC article.
References
-
- Alvarez V, Coto E, Setién F, González-Roces S, López-Larrea C. (1996) Molecular evolution of the N-formyl peptide and C5a receptors in non-human primates. Immunogenetics 44:446–452 - PubMed
-
- Ashwood V, Brownhill V, Higginbottom M, Horwell DC, Hughes J, Lewthwaite RA, McKnight AT, Pinnock RD, Pritchard MC, Suman-Chauhan N, et al. (1998) PD176252—the first high affinity non-peptide gastrin-releasing peptide (BB2) receptor antagonist. Bioorg Med Chem Lett 8:2589–2594 - PubMed
-
- Bae YS, Lee HY, Jo EJ, Kim JI, Kang HK, Ye RD, Kwak JY, Ryu SH. (2004) Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J Immunol 173:607–614 - PubMed
-
- Bae YS, Park JC, He R, Ye RD, Kwak JY, Suh PG, Ho Ryu S. (2003a) Differential signaling of formyl peptide receptor-like 1 by Trp-Lys-Tyr-Met-Val-Met-CONH2 or lipoxin A4 in human neutrophils. Mol Pharmacol 64:721–730 - PubMed
-
- Bae YS, Yi HJ, Lee HY, Jo EJ, Kim JI, Lee TG, Ye RD, Kwak JY, Ryu SH. (2003b) Differential activation of formyl peptide receptor-like 1 by peptide ligands. J Immunol 171:6807–6813 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

