Cinacalcet HCl reduces hypercalcemia in primary hyperparathyroidism across a wide spectrum of disease severity
- PMID: 20943783
- PMCID: PMC3203649
- DOI: 10.1210/jc.2010-1221
Cinacalcet HCl reduces hypercalcemia in primary hyperparathyroidism across a wide spectrum of disease severity
Abstract
Context: Primary hyperparathyroidism (PHPT) is characterized by elevated serum calcium (Ca) and increased PTH concentrations.
Objective: The objective of the investigation was to establish the efficacy of cinacalcet in reducing serum Ca in patients with PHPT across a wide spectrum of disease severity.
Design and setting: The study was a pooled analysis of data from three multicenter clinical trials of cinacalcet in PHPT.
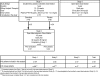
Patients: Patients were grouped into three disease categories for analysis based on the following: 1) history of failed parathyroidectomy (n = 29); 2) meeting one or more criteria for parathyroidectomy but without prior surgery (n = 37); and 3) mild asymptomatic PHPT without meeting criteria for either above category (n = 15).
Intervention: The intervention in this study was treatment with cinacalcet for up to 4.5 yr.
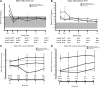
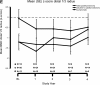
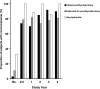
Outcomes: Measurements in the study included serum Ca, PTH, phosphate, and bone-specific alkaline phosphatase, and areal bone mineral density (aBMD). Vital signs, safety biochemical and hematological indices, and adverse events were monitored throughout the study period.
Results: The extent of cinacalcet-induced serum Ca reduction, proportion of patients achieving normal serum Ca (≤10.3 mg/dl), reduction in serum PTH, and increase in serum phosphate were similar across all three categories. Except for decreased aBMD at the total femur indicated for parathyroidectomy group at 1 yr, no significant changes in aBMD occurred. The efficacy of cinacalcet was maintained for up to 4.5 yr of follow-up. AEs were mild and similar across the three categories.
Conclusions: Cinacalcet is equally effective in the medical management of PHPT patients across a broad spectrum of disease severity, and overall cinacalcet is well tolerated.
Trial registration: ClinicalTrials.gov NCT00001859 NCT00037518 NCT00936988.
Figures
References
-
- Bilezikian JP, Potts Jr JT, Fuleihan Gel-H, Kleerekoper M, Neer R, Peacock M, Rastad J, Silverberg SJ, Udelsman R, Wells SA 2002 Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 87:5353–5361 - PubMed
-
- Eastell R, Arnold A, Brandi ML, Brown EM, D'Amour P, Hanley DA, Rao DS, Rubin MR, Goltzman D, Silverberg SJ, Marx SJ, Peacock M, Mosekilde L, Bouillon R, Lewiecki EM 2009 Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab 94:340–350 - PubMed
-
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP 1999 A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

