Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2
- PMID: 20943790
- PMCID: PMC3038480
- DOI: 10.1210/jc.2010-0692
Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2
Abstract
Context: Initiating factors leading to production of adrenal androgens are poorly defined. Cortisol is present in high concentrations within the adrenal gland, and its production rises with growth during childhood.
Objective: Our aim was to characterize the effect of cortisol and other glucocorticoids on androgen secretion from a human adrenocortical cell line and from nonadrenal cells transfected with CYP17A1 or HSD3B2.
Design/setting: This study was performed in cultured cells, at an academic medical center.
Methods: The effects of cortisol upon steroid production in human adrenal NCI-H295R cells were measured by immunoassay, tandem mass spectrometry, and thin-layer chromatography. The effects of cortisol upon the activities of 17, 20 lyase and 3βHSD2 were measured in NCI-H295R cells and in transfected COS-7 cells.
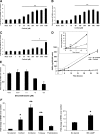
Results: Cortisol markedly and rapidly stimulated dehydroepiandrosterone (DHEA) in a dose-dependent manner at cortisol concentrations ≥50 μM. Cortisone and 11-deoxycortisol were also potent stimulators of DHEA secretion, whereas prednisolone and dexamethasone were not. Treatment with cortisol did not affect expression of CYP17A1 or HSD3B2 mRNAs. Stimulation of DHEA secretion by cortisol was associated with competitive inhibition of 3βHSD2 activity.
Conclusions: Cortisol inhibits 3βHSD2 activity in adrenal cells and in COS-7 cells transfected with HSD3B2. Thus, it is possible that intraadrenal cortisol may participate in the regulation of adrenal DHEA secretion through inhibition of 3βHSD2. We hypothesize that a rise in intraadrenal cortisol during childhood growth may lead to inhibition of 3βHSD2 activity and contribute to the initiation of adrenarche.
Figures
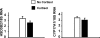


Similar articles
-
A New Model for Adrenarche: Inhibition of 3β-Hydroxysteroid Dehydrogenase Type 2 by Intra-Adrenal Cortisol.Horm Res Paediatr. 2018;89(5):311-319. doi: 10.1159/000488777. Epub 2018 May 30. Horm Res Paediatr. 2018. PMID: 29847819 Free PMC article. Review.
-
Assessment of the potential of polyphenols as a CYP17 inhibitor free of adverse corticosteroid elevation.Biochem Pharmacol. 2014 Aug 1;90(3):288-96. doi: 10.1016/j.bcp.2014.05.013. Epub 2014 May 27. Biochem Pharmacol. 2014. PMID: 24875446
-
1alpha,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells.Biochim Biophys Acta. 2010 Sep;1801(9):1056-62. doi: 10.1016/j.bbalip.2010.04.009. Epub 2010 Apr 24. Biochim Biophys Acta. 2010. PMID: 20420936
-
Leukemia inhibitory factor as a regulator of steroidogenesis in human NCI-H295R adrenocortical cells.J Endocrinol. 2008 Dec;199(3):435-44. doi: 10.1677/JOE-08-0377. Epub 2008 Sep 16. J Endocrinol. 2008. PMID: 18796549
-
Dissecting human adrenal androgen production.Trends Endocrinol Metab. 2002 Aug;13(6):234-9. doi: 10.1016/s1043-2760(02)00609-4. Trends Endocrinol Metab. 2002. PMID: 12128283 Review.
Cited by
-
The steroid metabolome of adrenarche.J Endocrinol. 2012 Aug;214(2):133-43. doi: 10.1530/JOE-12-0183. Epub 2012 Jun 19. J Endocrinol. 2012. PMID: 22715193 Free PMC article. Review.
-
A New Model for Adrenarche: Inhibition of 3β-Hydroxysteroid Dehydrogenase Type 2 by Intra-Adrenal Cortisol.Horm Res Paediatr. 2018;89(5):311-319. doi: 10.1159/000488777. Epub 2018 May 30. Horm Res Paediatr. 2018. PMID: 29847819 Free PMC article. Review.
-
Adrenal androgens and androgen precursors-definition, synthesis, regulation and physiologic actions.Compr Physiol. 2014 Oct;4(4):1369-81. doi: 10.1002/cphy.c140006. Compr Physiol. 2014. PMID: 25428847 Free PMC article. Review.
-
Association between Body Mass Index and Serum Dehydroepiandrosterone Sulfate Level in 8-Year-Old Girls.J Obes Metab Syndr. 2018 Jun 30;27(2):110-116. doi: 10.7570/jomes.2018.27.2.110. J Obes Metab Syndr. 2018. PMID: 31089550 Free PMC article.
-
Regulation of human 3β-hydroxysteroid dehydrogenase type 2 by adrenal corticosteroids and product-feedback by androstenedione in human adrenarche.J Pharmacol Exp Ther. 2015 Jan;352(1):67-76. doi: 10.1124/jpet.114.219550. Epub 2014 Oct 29. J Pharmacol Exp Ther. 2015. PMID: 25355646 Free PMC article.
References
-
- Talbot NR, Butler AM, Berman RA, Rodriguez PM, MacLachlan EA 1943 Excretion of 17-ketosteroids by normal and by abnormal children. AMA Am J Dis Child 65:364–375
-
- Palmert MR, Hayden DL, Mansfield MJ, Crigler Jr JF, Crowley Jr WF, Chandler DW, Boepple PA 2001 The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab 86:4536–4542 - PubMed
-
- Remer T, Boye KR, Hartmann MF, Wudy SA 2005 Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 90:2015–2021 - PubMed
-
- Ibáñez L, Díaz R, López-Bermejo A, Marcos MV 2009 Clinical spectrum of premature pubarche: links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr Metab Disord 10:63–76 - PubMed
-
- Miller WL 2009 Androgen synthesis in adrenarche. Rev Endocr Metab Disord 10:3–17 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

