Metabolomics identifies novel Hnf1alpha-dependent physiological pathways in vivo
- PMID: 20943816
- PMCID: PMC2999475
- DOI: 10.1210/me.2010-0130
Metabolomics identifies novel Hnf1alpha-dependent physiological pathways in vivo
Abstract
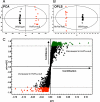
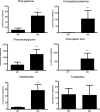
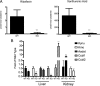
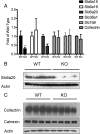
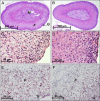
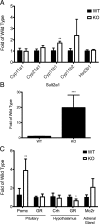
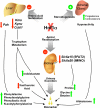
Mutations in the HNF1A gene cause maturity-onset diabetes of the young type 3, one of the most common genetic causes of non-insulin-dependent (type 2) diabetes mellitus. Although the whole-body Hnf1a-null mouse recapitulates the low insulin levels and high blood glucose observed in human maturity-onset diabetes of the young type 3 patients, these mice also suffer from Laron dwarfism and aminoaciduria, suggesting a role for hepatocyte nuclear factor 1α (Hnf1α) in pathophysiologies distinct from non-insulin-dependent (type 2) diabetes mellitus. In an effort to identify pathways associated with inactivation of Hnf1α, an ultraperformance liquid chromatography coupled to mass spectrometry-based metabolomics study was conducted on urine samples from wild-type and Hnf1a-null mice. An increase in phenylalanine metabolites is in agreement with the known regulation of the phenylalanine hydroxylase gene by Hnf1α. This metabolomic approach also identified urinary biomarkers for three tissue-specific dysfunctions previously unassociated with Hnf1α function. 1) Elevated indolelactate coupled to decreased xanthurenic acid also indicated defects in the indole and kynurenine pathways of tryptophan metabolism, respectively. 2) An increase in the neutral amino acid proline in the urine of Hnf1a-null mice correlated with loss of renal apical membrane transporters of the Slc6a family. 3) Further investigation into the mechanism of aldosterone increase revealed an overactive adrenal gland in Hnf1a-null mice possibly due to inhibition of negative feedback regulation. Although the phenotype of the Hnf1a-null mouse is complex, metabolomics has opened the door to investigation of several physiological systems in which Hnf1α may be a critical regulatory component.
Figures
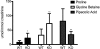
References
-
- Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Bell GI, et al. 1996 Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 384:455–458 - PubMed
-
- Shih DQ, Screenan S, Munoz KN, Philipson L, Pontoglio M, Yaniv M, Polonsky KS, Stoffel M 2001 Loss of HNF-1α function in mice leads to abnormal expression of genes involved in pancreatic islet development and metabolism. Diabetes 50:2472–2480 - PubMed
-
- Dukes ID, Sreenan S, Roe MW, Levisetti M, Zhou YP, Ostrega D, Bell GI, Pontoglio M, Yaniv M, Philipson L, Polonsky KS 1998 Defective pancreatic β-cell glycolytic signaling in hepatocyte nuclear factor-1α-deficient mice. J Biol Chem 273:24457–24464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

