Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster
- PMID: 20943882
- PMCID: PMC3008188
- DOI: 10.1128/CVI.00117-10
Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster
Abstract
This study was conducted to evaluate the effect of a reduced-dose 7-valent pneumococcal conjugate vaccine (PCV) primary series followed by a 23-valent pneumococcal polysaccharide vaccine (23vPPS) booster on nasopharyngeal (NP) pneumococcal carriage. For this purpose, Fijian infants aged 6 weeks were randomized to receive 0, 1, 2, or 3 PCV doses. Within each group, half received 23vPPS at 12 months. NP swabs were taken at 6, 9, 12, and 17 months and were cultured for Streptococcus pneumoniae. Isolates were serotyped by multiplex PCR and a reverse line blot assay. There were no significant differences in PCV vaccine type (VT) carriage between the 3- and 2-dose groups at 12 months. NP VT carriage was significantly higher (P, <0.01) in the unvaccinated group than in the 3-dose group at the age of 9 months. There appeared to be a PCV dose effect in the cumulative proportion of infants carrying the VT, with less VT carriage occurring with more doses of PCV. Non-PCV serotype (NVT) carriage rates were similar for all PCV groups. When groups were pooled by receipt or nonreceipt of 23vPPS at 12 months, there were no differences in pneumococcal, VT, or NVT carriage rates between the 2 groups at the age of 17 months. In conclusion, there appeared to be a PCV dose effect on VT carriage, with less VT carriage occurring with more doses of PCV. By the age of 17 months, NVT carriage rates were similar for all groups. 23vPPS had no impact on carriage, despite the substantial boosts in antibody levels.
Trial registration: ClinicalTrials.gov NCT00170612.
Figures
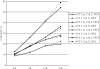
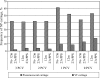
References
-
- Reference deleted.
-
- Cheung, Y. B., S. M. Zaman, E. D. Nsekpong, C. A. Van Beneden, R. A. Adegbola, B. Greenwood, and F. T. Cutts. 2009. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian children who participated in a 9-valent pneumococcal conjugate vaccine trial and in their younger siblings. Pediatr. Infect. Dis. J. 28:990-995. - PubMed
-
- Christensen, P., B. Hovelius, K. Prellner, C. Rosen, K. K. Christensen, D. N. Kurl, L. Larsson, A. Stjernquist-Desatnik, and C. Schalen. 1985. Effects of pneumococcal vaccination on tonsillo-pharyngitis and upper respiratory tract flora. Int. Arch. Allergy Appl. Immunol. 78:161-166. - PubMed
-
- Cohen, R., C. Levy, F. de La Rocque, N. Gelbert, A. Wollner, B. Fritzell, E. Bonnet, R. Tetelboum, and E. Varon. 2006. Impact of pneumococcal conjugate vaccine and of reduction of antibiotic use on nasopharyngeal carriage of nonsusceptible pneumococci in children with acute otitis media. Pediatr. Infect. Dis. J. 25:1001-1007. - PubMed
-
- Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

