Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells
- PMID: 20943904
- PMCID: PMC2968711
- DOI: 10.1523/JNEUROSCI.3226-10.2010
Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells
Abstract
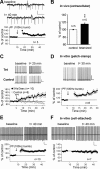
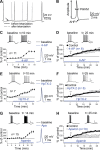
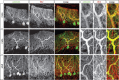
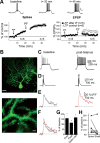
Synaptic gain control and information storage in neural networks are mediated by alterations in synaptic transmission, such as in long-term potentiation (LTP). Here, we show using both in vitro and in vivo recordings from the rat cerebellum that tetanization protocols for the induction of LTP at parallel fiber (PF)-to-Purkinje cell synapses can also evoke increases in intrinsic excitability. This form of intrinsic plasticity shares with LTP a requirement for the activation of protein phosphatases 1, 2A, and 2B for induction. Purkinje cell intrinsic plasticity resembles CA1 hippocampal pyramidal cell intrinsic plasticity in that it requires activity of protein kinase A (PKA) and casein kinase 2 (CK2) and is mediated by a downregulation of SK-type calcium-sensitive K conductances. In addition, Purkinje cell intrinsic plasticity similarly results in enhanced spine calcium signaling. However, there are fundamental differences: first, while in the hippocampus increases in excitability result in a higher probability for LTP induction, intrinsic plasticity in Purkinje cells lowers the probability for subsequent LTP induction. Second, intrinsic plasticity raises the spontaneous spike frequency of Purkinje cells. The latter effect does not impair tonic spike firing in the target neurons of inhibitory Purkinje cell projections in the deep cerebellar nuclei, but lowers the Purkinje cell signal-to-noise ratio, thus reducing the PF readout. These observations suggest that intrinsic plasticity accompanies LTP of active PF synapses, while it reduces at weaker, nonpotentiated synapses the probability for subsequent potentiation and lowers the impact on the Purkinje cell output.
Figures





Similar articles
-
Long-Term Depression of Intrinsic Excitability Accompanied by Synaptic Depression in Cerebellar Purkinje Cells.J Neurosci. 2017 Jun 7;37(23):5659-5669. doi: 10.1523/JNEUROSCI.3464-16.2017. Epub 2017 May 11. J Neurosci. 2017. PMID: 28495974 Free PMC article.
-
Climbing fiber-evoked endocannabinoid signaling heterosynaptically suppresses presynaptic cerebellar long-term potentiation.J Neurosci. 2006 Aug 9;26(32):8289-94. doi: 10.1523/JNEUROSCI.0805-06.2006. J Neurosci. 2006. PMID: 16899723 Free PMC article.
-
β-Adrenergic Receptors/Epac Signaling Increases the Size of the Readily Releasable Pool of Synaptic Vesicles Required for Parallel Fiber LTP.J Neurosci. 2020 Nov 4;40(45):8604-8617. doi: 10.1523/JNEUROSCI.0716-20.2020. Epub 2020 Oct 12. J Neurosci. 2020. PMID: 33046543 Free PMC article.
-
Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses.Neuron. 2006 Oct 19;52(2):227-38. doi: 10.1016/j.neuron.2006.09.032. Neuron. 2006. PMID: 17046686 Review.
-
SK2 channel expression and function in cerebellar Purkinje cells.J Physiol. 2011 Jul 15;589(Pt 14):3433-40. doi: 10.1113/jphysiol.2011.205823. Epub 2011 Apr 26. J Physiol. 2011. PMID: 21521760 Free PMC article. Review.
Cited by
-
Transcranial direct current stimulation of cerebellum alters spiking precision in cerebellar cortex: A modeling study of cellular responses.PLoS Comput Biol. 2021 Dec 9;17(12):e1009609. doi: 10.1371/journal.pcbi.1009609. eCollection 2021 Dec. PLoS Comput Biol. 2021. PMID: 34882680 Free PMC article.
-
Intrinsic excitement in cerebellar nuclei neurons during learning.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):9824-9826. doi: 10.1073/pnas.1813866115. Epub 2018 Sep 14. Proc Natl Acad Sci U S A. 2018. PMID: 30217888 Free PMC article. No abstract available.
-
Neural ensembles: role of intrinsic excitability and its plasticity.Front Cell Neurosci. 2024 Jul 31;18:1440588. doi: 10.3389/fncel.2024.1440588. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39144154 Free PMC article. Review.
-
Purkinje cell intrinsic excitability increases after synaptic long term depression.J Neurophysiol. 2016 Sep 1;116(3):1208-17. doi: 10.1152/jn.00369.2016. Epub 2016 Jun 15. J Neurophysiol. 2016. PMID: 27306677 Free PMC article.
-
Impact of NMDA Receptor Overexpression on Cerebellar Purkinje Cell Activity and Motor Learning.eNeuro. 2018 Feb 12;5(1):ENEURO.0270-17.2018. doi: 10.1523/ENEURO.0270-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29464191 Free PMC article.
References
-
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
