RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus
- PMID: 20943920
- PMCID: PMC6633732
- DOI: 10.1523/JNEUROSCI.1567-10.2010
RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus
Abstract
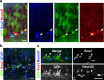
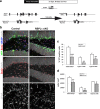
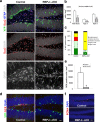
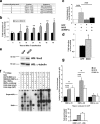
The generation of new neurons from neural stem cells in the adult hippocampal dentate gyrus contributes to learning and mood regulation. To sustain hippocampal neurogenesis throughout life, maintenance of the neural stem cell pool has to be tightly controlled. We found that the Notch/RBPJκ-signaling pathway is highly active in neural stem cells of the adult mouse hippocampus. Conditional inactivation of RBPJκ in neural stem cells in vivo resulted in increased neuronal differentiation of neural stem cells in the adult hippocampus at an early time point and depletion of the Sox2-positive neural stem cell pool and suppression of hippocampal neurogenesis at a later time point. Moreover, RBPJκ-deficient neural stem cells displayed impaired self-renewal in vitro and loss of expression of the transcription factor Sox2. Interestingly, we found that Notch signaling increases Sox2 promoter activity and Sox2 expression in adult neural stem cells. In addition, activated Notch and RBPJκ were highly enriched on the Sox2 promoter in adult hippocampal neural stem cells, thus identifying Sox2 as a direct target of Notch/RBPJκ signaling. Finally, we found that overexpression of Sox2 can rescue the self-renewal defect in RBPJκ-deficient neural stem cells. These results identify RBPJκ-dependent pathways as essential regulators of adult neural stem cell maintenance and suggest that the actions of RBPJκ are, at least in part, mediated by control of Sox2 expression.
Figures



References
-
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. - PubMed
-
- Aizawa K, Ageyama N, Terao K, Hisatsune T. Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging. 2010 in press. - PubMed
-
- Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. - PubMed
-
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
