Analysis of the astray/robo2 zebrafish mutant reveals that degenerating tracts do not provide strong guidance cues for regenerating optic axons
- PMID: 20943924
- PMCID: PMC3058139
- DOI: 10.1523/JNEUROSCI.3846-10.2010
Analysis of the astray/robo2 zebrafish mutant reveals that degenerating tracts do not provide strong guidance cues for regenerating optic axons
Abstract
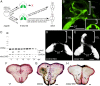
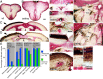
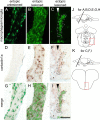
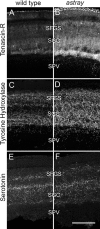
During formation of the optic projection in astray/robo2 mutant zebrafish, optic axons exhibit rostrocaudal pathfinding errors, ectopic midline crossing and increased terminal arbor size. Here we show that these errors persist into adulthood, even when robo2 function is conditionally reduced only during initial formation of the optic projection. Adult errors include massive ectopic optic tracts in the telencephalon. During optic nerve regeneration in astray/robo2 animals, these tracts are not repopulated and ectopic midline crossing is reduced compared with unlesioned mutants. This is despite a comparable macrophage/microglial response and upregulation of contactin1a in oligodendrocytes of entopic and ectopic tracts. However, other errors, such as expanded termination areas and ectopic growth into the tectum, were frequently recommitted by regenerating optic axons. Retinal ganglion cells with regenerating axons reexpress robo2 and expression of slit ligands is maintained in some areas of the adult optic pathway. However, slit expression is reduced rostral and caudal to the chiasm, compared with development and ubiquitous overexpression of Slit2 did not elicit major pathfinding phenotypes. This shows that (1) there is not an efficient correction mechanism for large-scale pathfinding errors of optic axons during development; (2) degenerating tracts do not provide a strong guidance cue for regenerating optic axons in the adult CNS, unlike the PNS; and (3) robo2 is less important for pathfinding of optic axons during regeneration than during development.
Figures


Similar articles
-
Robo2 Drives Target-Selective Peripheral Nerve Regeneration in Response to Glia-Derived Signals.J Neurosci. 2022 Feb 2;42(5):762-776. doi: 10.1523/JNEUROSCI.1528-21.2021. Epub 2021 Dec 16. J Neurosci. 2022. PMID: 34916258 Free PMC article.
-
Pathfinding and error correction by retinal axons: the role of astray/robo2.Neuron. 2002 Jan 17;33(2):205-17. doi: 10.1016/s0896-6273(01)00579-7. Neuron. 2002. PMID: 11804569
-
astray, a zebrafish roundabout homolog required for retinal axon guidance.Science. 2001 Apr 20;292(5516):507-10. doi: 10.1126/science.1059496. Science. 2001. PMID: 11313496
-
Growth and pathfinding of regenerating axons in the optic projection of adult fish.J Neurosci Res. 2007 Sep;85(12):2793-9. doi: 10.1002/jnr.21121. J Neurosci Res. 2007. PMID: 17131420 Review.
-
Traumatology of the optic nerve and contribution of crystallins to axonal regeneration.Cell Tissue Res. 2012 Jul;349(1):49-69. doi: 10.1007/s00441-012-1442-4. Epub 2012 May 26. Cell Tissue Res. 2012. PMID: 22638995 Review.
Cited by
-
Robo2--slit and Dcc--netrin1 coordinate neuron axonal pathfinding within the embryonic axon tracts.J Neurosci. 2012 Sep 5;32(36):12589-602. doi: 10.1523/JNEUROSCI.6518-11.2012. J Neurosci. 2012. PMID: 22956848 Free PMC article.
-
Retrograde labeling of retinal ganglion cells in adult zebrafish with fluorescent dyes.J Vis Exp. 2014 May 3;(87):50987. doi: 10.3791/50987. J Vis Exp. 2014. PMID: 24837333 Free PMC article.
-
Cell proliferation and apoptosis in optic nerve and brain integration centers of adult trout Oncorhynchus mykiss after optic nerve injury.Neural Regen Res. 2016 Apr;11(4):578-90. doi: 10.4103/1673-5374.180742. Neural Regen Res. 2016. PMID: 27212918 Free PMC article.
-
In vivo nerve-macrophage interactions following peripheral nerve injury.J Neurosci. 2012 Mar 14;32(11):3898-909. doi: 10.1523/JNEUROSCI.5225-11.2012. J Neurosci. 2012. PMID: 22423110 Free PMC article.
-
Viral vector-based improvement of optic nerve regeneration: characterization of individual axons' growth patterns and synaptogenesis in a visual target.Gene Ther. 2015 Oct;22(10):811-21. doi: 10.1038/gt.2015.51. Epub 2015 May 25. Gene Ther. 2015. PMID: 26005861 Free PMC article.
References
-
- Ankerhold R, Leppert CA, Bastmeyer M, Stuermer CA. E587 antigen is upregulated by goldfish oligodendrocytes after optic nerve lesion and supports retinal axon regeneration. Glia. 1998;23:257–270. - PubMed
-
- Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair—what can it achieve? Nat Clin Pract Neurol. 2007;3:152–161. - PubMed
-
- Becker CG, Becker T. Growth and pathfinding of regenerating axons in the optic projection of adult fish. J Neurosci Res. 2007;85:2793–2799. - PubMed
-
- Becker CG, Meyer RL, Becker T. Gradients of ephrin-A2 and ephrin-A5b mRNA during retinotopic regeneration of the optic projection in adult zebrafish. J Comp Neurol. 2000;427:469–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
