Arsenic-induced SUMO-dependent recruitment of RNF4 into PML nuclear bodies
- PMID: 20943951
- PMCID: PMC2993750
- DOI: 10.1091/mbc.E10-05-0449
Arsenic-induced SUMO-dependent recruitment of RNF4 into PML nuclear bodies
Abstract
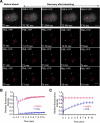
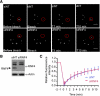
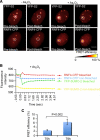
In acute promyelocytic leukemia (APL), the promyelocytic leukemia (PML) protein is fused to the retinoic acid receptor alpha (RAR). Arsenic is an effective treatment for this disease as it induces SUMO-dependent ubiquitin-mediated proteasomal degradation of the PML-RAR fusion protein. Here we analyze the nuclear trafficking dynamics of PML and its SUMO-dependent ubiquitin E3 ligase, RNF4 in response to arsenic. After administration of arsenic, PML immediately transits into nuclear bodies where it undergoes SUMO modification. This initial recruitment of PML into nuclear bodies is not dependent on RNF4, but RNF4 quickly follows PML into the nuclear bodies where it is responsible for ubiquitylation of SUMO-modified PML and its degradation by the proteasome. While arsenic restricts the mobility of PML, FRAP analysis indicates that RNF4 continues to rapidly shuttle into PML nuclear bodies in a SUMO-dependent manner. Under these conditions FRET studies indicate that RNF4 interacts with SUMO in PML bodies but not directly with PML. These studies indicate that arsenic induces the rapid reorganization of the cell nucleus by SUMO modification of nuclear body-associated PML and uptake of the ubiquitin E3 ligase RNF4 leading to the ubiquitin-mediated degradation of PML.
Figures
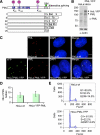
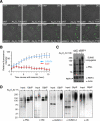

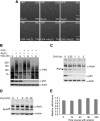
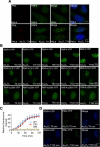


References
-
- Bernardi R., Pandolfi P. P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 2007;8:1006–1016. - PubMed
-
- de The H., Chomienne C., Lanotte M., Degos L., Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–561. - PubMed
-
- de The H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. - PubMed
-
- Dyck J.A., Maul G. G., Miller W. H., Chen J. D., Kakizuka A., Evans R. M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

