Cystathionine β-synthase and cystathionine γ-lyase double gene transfer ameliorate homocysteine-mediated mesangial inflammation through hydrogen sulfide generation
- PMID: 20943958
- PMCID: PMC3023186
- DOI: 10.1152/ajpcell.00143.2010
Cystathionine β-synthase and cystathionine γ-lyase double gene transfer ameliorate homocysteine-mediated mesangial inflammation through hydrogen sulfide generation
Abstract
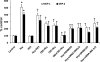
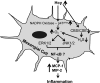
Elevated level of homocysteine (Hcy) induces chronic inflammation in vascular bed, including glomerulus, and promotes glomerulosclerosis. In this study we investigated in vitro mechanism of Hcy-mediated monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-2 (MIP-2) induction and determined the regulatory role of hydrogen sulfide (H₂S) to ameliorate inflammation. Mouse glomerular mesangial cells (MCs) were incubated with Hcy (75 μM) and supplemented with vehicle or with H₂S (30 μM, in the form of NaHS). Inflammatory molecules MCP-1 and MIP-2 were measured by ELISA. Cellular capability to generate H₂S was measured by colorimetric chemical method. To enhance endogenous production of H₂S and better clearance of Hcy, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) genes were delivered to the cells. Oxidative NAD(P)H p47(phox) was measured by Western blot analysis and immunostaining. Phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and c-Jun NH₂-terminal kinase (JNK1/2) were measured by Western blot analysis. Our results demonstrated that Hcy upregulated inflammatory molecules MCP-1 and MIP-2, whereas endogenous production of H₂S was attenuated. H₂S treatment as well as CBS and CSE doubly cDNA overexpression markedly reduced Hcy-induced upregulation of MCP-1 and MIP-2. Hcy-induced upregulation of oxidative p47(phox) was attenuated by H₂S supplementation and CBS/CSE overexpression as well. In addition to that we also detected Hcy-induced MCP-1 and MIP-2 induction was through phosphorylation of ERK1/2 and JNK1/2. Either H₂S supplementation or CBS and CSE doubly cDNA overexpression attenuated Hcy-induced phosphorylation of these two signaling molecules and diminished MCP-1 and MIP-2 expressions. Similar results were obtained by inhibition of ERK1/2 and JNK1/2 using pharmacological and small interferring RNA (siRNA) blockers. We conclude that H₂S plays a regulatory role in Hcy-induced mesangial inflammation and that ERK1/2 and JNK1/2 are two signaling pathways involved this process.
Figures





Similar articles
-
Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia.Am J Physiol Cell Physiol. 2012 Jul 1;303(1):C41-51. doi: 10.1152/ajpcell.00398.2011. Epub 2012 Apr 18. Am J Physiol Cell Physiol. 2012. PMID: 22517358 Free PMC article.
-
Dysregulation of cystathionine γ-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage.Cell Signal. 2013 Nov;25(11):2255-62. doi: 10.1016/j.cellsig.2013.07.010. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872072
-
Beneficial Role of Hydrogen Sulfide in Renal Ischemia Reperfusion Injury in Rats.Yonsei Med J. 2018 Oct;59(8):960-967. doi: 10.3349/ymj.2018.59.8.960. Yonsei Med J. 2018. PMID: 30187703 Free PMC article.
-
The role of the mitochondrial trans-sulfuration in cerebro-cardio renal dysfunction during trisomy down syndrome.Mol Cell Biochem. 2024 Apr;479(4):825-829. doi: 10.1007/s11010-023-04761-9. Epub 2023 May 17. Mol Cell Biochem. 2024. PMID: 37198322 Review.
-
Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-β- synthase (CBS) and cystathionine-γ-lyase (CSE).Inflamm Allergy Drug Targets. 2011 Apr;10(2):85-91. doi: 10.2174/187152811794776286. Inflamm Allergy Drug Targets. 2011. PMID: 21275900 Review.
Cited by
-
Homocysteine Triggers Inflammatory Responses in Macrophages through Inhibiting CSE-H2S Signaling via DNA Hypermethylation of CSE Promoter.Int J Mol Sci. 2015 Jun 3;16(6):12560-77. doi: 10.3390/ijms160612560. Int J Mol Sci. 2015. PMID: 26047341 Free PMC article.
-
Platelet-derived growth factor-BB induces cystathionine γ-lyase expression in rat mesangial cells via a redox-dependent mechanism.Br J Pharmacol. 2012 Aug;166(8):2231-42. doi: 10.1111/j.1476-5381.2012.01949.x. Br J Pharmacol. 2012. PMID: 22428706 Free PMC article.
-
The Pathophysiology of H2S in Renal Glomerular Diseases.Biomolecules. 2022 Jan 26;12(2):207. doi: 10.3390/biom12020207. Biomolecules. 2022. PMID: 35204708 Free PMC article. Review.
-
Homocysteine mediates transcriptional changes of the inflammatory pathway signature genes in human retinal pigment epithelial cells.Int J Ophthalmol. 2017 May 18;10(5):696-704. doi: 10.18240/ijo.2017.05.06. eCollection 2017. Int J Ophthalmol. 2017. PMID: 28546923 Free PMC article.
-
Downregulation of cystathionine β-synthase and cystathionine γ-lyase expression stimulates inflammation in kidney ischemia-reperfusion injury.Physiol Rep. 2014 Dec 24;2(12):e12251. doi: 10.14814/phy2.12251. Print 2014 Dec 1. Physiol Rep. 2014. PMID: 25539831 Free PMC article.
References
-
- Abe K, Watanabe Y, Saito H. Differential role of nitric oxide in long-term potentiation in the medial and lateral amygdala. Eur J Pharmacol 297: 43–46, 1996 - PubMed
-
- Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34: 573–585, 2008 - PubMed
-
- Cheung GT, Siow YL, OK Homocysteine stimulates monocyte chemoattractant protein-1 expression in mesangial cells via NF-kappaB activation. Can J Physiol Pharmacol 86: 88–96, 2008 - PubMed
-
- Choudhury GG, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abboud HE. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am J Physiol Renal Physiol 273: F931–F938, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

