Cytoplasmic poly(A) binding proteins regulate telomerase activity and cell growth in human papillomavirus type 16 E6-expressing keratinocytes
- PMID: 20943973
- PMCID: PMC3004306
- DOI: 10.1128/JVI.01377-10
Cytoplasmic poly(A) binding proteins regulate telomerase activity and cell growth in human papillomavirus type 16 E6-expressing keratinocytes
Abstract
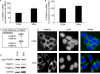
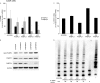
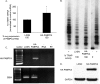
The high-risk human papillomavirus (HPV) E6 and E7 oncoproteins are critical to the immortalization of keratinocytes. HPV type 16 (HPV16) E6 interacts with endogenous proteins to activate hTERT, the catalytic subunit of telomerase, thus avoiding cellular senescence signals. NFX1-123, the longer splice variant of NFX1, interacts with HPV16 E6, as well as cytoplasmic poly(A) binding proteins 1 and 4 (PABPC1 and PABPC4). HPV16 E6 affects hTERT expression posttranscriptionally through NFX1-123, as NFX1-123 interacts with hTERT mRNA and stabilizes it, leading to greater telomerase activity. The PAM2 motif of NFX1-123, with which it binds PABPCs, is required for the posttranscriptional regulation of hTERT by HPV16 E6 and NFX1-123. There is increasing evidence that RNA and DNA viruses utilize RNA-processing proteins, and specifically PABPCs, in the normal virus life cycle, and there is also evidence that RNA-processing proteins are perturbed in cancers. Here, we show that PABPCs are critical in hTERT regulation by HPV16 E6. Although the amount and cellular localization of PABPCs were largely unchanged in cervical cancer cell lines with or without HPV16 and in human foreskin keratinocytes (HFKs) with or without HPV16 E6, knockdown of PABPCs decreased hTERT mRNA and telomerase activity and overexpression of PABPC4 increased these in HPV16 E6-expressing HFKs. In contrast, knockdown of PABPCs in C33A cells had no effect on hTERT mRNA or telomerase activity. Additionally, overexpression of PABPC4 and hTERT led to greater growth of cultured HPV16 E6-expressing HFKs. This is the first evidence that PABPCs have a targeted role in hTERT regulation leading to a growth advantage in cells expressing HPV16 E6.
Figures


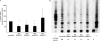
Similar articles
-
NFX1-123 is highly expressed in cervical cancer and increases growth and telomerase activity in HPV 16E6 expressing cells.Cancer Lett. 2019 May 1;449:106-113. doi: 10.1016/j.canlet.2019.02.024. Epub 2019 Feb 16. Cancer Lett. 2019. PMID: 30776478 Free PMC article.
-
NFX1-123 increases hTERT expression and telomerase activity posttranscriptionally in human papillomavirus type 16 E6 keratinocytes.J Virol. 2009 Jul;83(13):6446-56. doi: 10.1128/JVI.02556-08. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369336 Free PMC article.
-
NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells.J Virol. 2007 Apr;81(8):3786-96. doi: 10.1128/JVI.02007-06. Epub 2007 Jan 31. J Virol. 2007. PMID: 17267499 Free PMC article.
-
Activation of telomerase by HPVs.Virus Res. 2017 Mar 2;231:50-55. doi: 10.1016/j.virusres.2016.11.003. Epub 2016 Nov 15. Virus Res. 2017. PMID: 27863966 Review.
-
Post-Transcriptional Gene Regulation by HPV 16E6 and Its Host Protein Partners.Viruses. 2022 Jul 6;14(7):1483. doi: 10.3390/v14071483. Viruses. 2022. PMID: 35891463 Free PMC article. Review.
Cited by
-
The Molecular Interplay between Human Oncoviruses and Telomerase in Cancer Development.Cancers (Basel). 2022 Oct 26;14(21):5257. doi: 10.3390/cancers14215257. Cancers (Basel). 2022. PMID: 36358677 Free PMC article. Review.
-
NFX1-123 is highly expressed in cervical cancer and increases growth and telomerase activity in HPV 16E6 expressing cells.Cancer Lett. 2019 May 1;449:106-113. doi: 10.1016/j.canlet.2019.02.024. Epub 2019 Feb 16. Cancer Lett. 2019. PMID: 30776478 Free PMC article.
-
Molecular Mechanisms of MmuPV1 E6 and E7 and Implications for Human Disease.Viruses. 2022 Sep 28;14(10):2138. doi: 10.3390/v14102138. Viruses. 2022. PMID: 36298698 Free PMC article. Review.
-
HPV type 16 E6 and NFX1-123 augment JNK signaling to mediate keratinocyte differentiation and L1 expression.Virology. 2019 May;531:171-182. doi: 10.1016/j.virol.2019.03.008. Epub 2019 Mar 16. Virology. 2019. PMID: 30903928 Free PMC article.
-
Telomerase Induction in HPV Infection and Oncogenesis.Viruses. 2017 Jul 10;9(7):180. doi: 10.3390/v9070180. Viruses. 2017. PMID: 28698524 Free PMC article. Review.
References
-
- Albrecht, M., and T. Lengauer. 2004. Survey on the PABC recognition motif PAM2. Biochem. Biophys. Res. Commun. 316:129-138. - PubMed
-
- Auersperg, N. 1964. Long-term cultivation of hypodiploid human tumor cells. J. Natl. Cancer Inst. 32:135-163. - PubMed
-
- Bartz, S. R., and M. A. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337-342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

