Nodavirus-induced membrane rearrangement in replication complex assembly requires replicase protein a, RNA templates, and polymerase activity
- PMID: 20943974
- PMCID: PMC3004334
- DOI: 10.1128/JVI.01495-10
Nodavirus-induced membrane rearrangement in replication complex assembly requires replicase protein a, RNA templates, and polymerase activity
Abstract
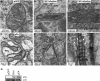
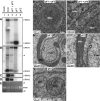
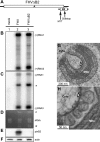
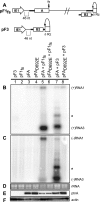
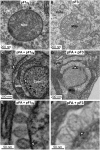
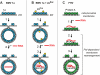
Positive-strand RNA [(+)RNA] viruses invariably replicate their RNA genomes on modified intracellular membranes. In infected Drosophila cells, Flock House nodavirus (FHV) RNA replication complexes form on outer mitochondrial membranes inside ∼50-nm, virus-induced spherular invaginations similar to RNA replication-linked spherules induced by many (+)RNA viruses at various membranes. To better understand replication complex assembly, we studied the mechanisms of FHV spherule formation. FHV has two genomic RNAs; RNA1 encodes multifunctional RNA replication protein A and RNA interference suppressor protein B2, while RNA2 encodes the capsid proteins. Expressing genomic RNA1 without RNA2 induced mitochondrial spherules indistinguishable from those in FHV infection. RNA1 mutation showed that protein B2 was dispensable and that protein A was the only FHV protein required for spherule formation. However, expressing protein A alone only "zippered" together the surfaces of adjacent mitochondria, without inducing spherules. Thus, protein A is necessary but not sufficient for spherule formation. Coexpressing protein A plus a replication-competent FHV RNA template induced RNA replication in trans and membrane spherules. Moreover, spherules were not formed when replicatable FHV RNA templates were expressed with protein A bearing a single, polymerase-inactivating amino acid change or when wild-type protein A was expressed with a nonreplicatable FHV RNA template. Thus, unlike many (+)RNA viruses, the membrane-bounded compartments in which FHV RNA replication occurs are not induced solely by viral protein(s) but require viral RNA synthesis. In addition to replication complex assembly, the results have implications for nodavirus interaction with cell RNA silencing pathways and other aspects of virus control.
Figures


Similar articles
-
5' cis elements direct nodavirus RNA1 recruitment to mitochondrial sites of replication complex formation.J Virol. 2009 Apr;83(7):2976-88. doi: 10.1128/JVI.02040-08. Epub 2009 Jan 14. J Virol. 2009. PMID: 19144713 Free PMC article.
-
Nodavirus RNA replication protein a induces membrane association of genomic RNA.J Virol. 2007 May;81(9):4633-44. doi: 10.1128/JVI.02267-06. Epub 2007 Feb 14. J Virol. 2007. PMID: 17301137 Free PMC article.
-
Flock house virus RNA polymerase initiates RNA synthesis de novo and possesses a terminal nucleotidyl transferase activity.PLoS One. 2014 Jan 23;9(1):e86876. doi: 10.1371/journal.pone.0086876. eCollection 2014. PLoS One. 2014. PMID: 24466277 Free PMC article.
-
Recent insights into the biology and biomedical applications of Flock House virus.Cell Mol Life Sci. 2008 Sep;65(17):2675-87. doi: 10.1007/s00018-008-8037-y. Cell Mol Life Sci. 2008. PMID: 18516498 Free PMC article. Review.
-
Bromovirus-induced remodeling of host membranes during viral RNA replication.Curr Opin Virol. 2014 Dec;9:104-10. doi: 10.1016/j.coviro.2014.09.018. Epub 2014 Oct 16. Curr Opin Virol. 2014. PMID: 25462441 Review.
Cited by
-
Cryo-electron tomography reveals novel features of a viral RNA replication compartment.Elife. 2017 Jun 27;6:e25940. doi: 10.7554/eLife.25940. Elife. 2017. PMID: 28653620 Free PMC article.
-
Inspirations on Virus Replication and Cell-to-Cell Movement from Studies Examining the Cytopathology Induced by Lettuce infectious yellows virus in Plant Cells.Front Plant Sci. 2017 Sep 27;8:1672. doi: 10.3389/fpls.2017.01672. eCollection 2017. Front Plant Sci. 2017. PMID: 29021801 Free PMC article. Review.
-
Spherules and IBV.Bioengineered. 2014 Sep-Oct;5(5):288-92. doi: 10.4161/bioe.29323. Bioengineered. 2014. PMID: 25482229 Free PMC article.
-
Morphogenesis of Endoplasmic Reticulum Membrane-Invaginated Vesicles during Beet Black Scorch Virus Infection: Role of Auxiliary Replication Protein and New Implications of Three-Dimensional Architecture.J Virol. 2015 Jun;89(12):6184-95. doi: 10.1128/JVI.00401-15. Epub 2015 Apr 1. J Virol. 2015. PMID: 25833056 Free PMC article.
-
Identification and Characterization of Two Novel Noda-like Viruses from Rice Plants Showing the Dwarfing Symptom.Viruses. 2022 May 27;14(6):1159. doi: 10.3390/v14061159. Viruses. 2022. PMID: 35746632 Free PMC article.
References
-
- Albariño, C. G., B. D. Price, L. D. Eckerle, and L. A. Ball. 2001. Characterization and template properties of RNA dimers generated during Flock House virus RNA replication. Virology 289:269-282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

