Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms
- PMID: 20943977
- PMCID: PMC3004348
- DOI: 10.1128/JVI.01328-10
Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms
Abstract
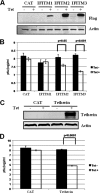
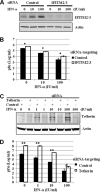
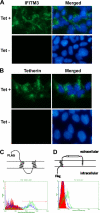
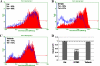
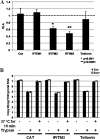
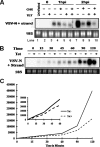
Tetherin and IFITM3 are recently identified interferon-induced cellular proteins that restrict infections by retroviruses and filoviruses and of influenza virus and flaviviruses, respectively. In our efforts to further explore their antiviral activities against other viruses and determine their antiviral mechanisms, we found that the two antiviral proteins potently inhibit the infection of vesicular stomatitis virus (VSV), a prototype member of the Rhabdoviridae family. Taking advantage of this well-studied virus infection system, we show that although both tetherin and IFITM3 are plasma membrane proteins, tetherin inhibits virion particle release from infected cells, while IFITM3 disrupts an early event after endocytosis of virion particles but before primary transcription of incoming viral genomes. Furthermore, we demonstrate that both the N-terminal 21 amino acid residues and C-terminal transmembrane region of IFITM3 are required for its antiviral activity. Collectively, our work sheds light on the mechanisms by which tetherin and IFITM3 restrict infection with rhabdoviruses and possibly other pathogenic viruses.
Figures



References
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Andreu, P., S. Colnot, C. Godard, P. Laurent-Puig, D. Lamarque, A. Kahn, C. Perret, and B. Romagnolo. 2006. Identification of the IFITM family as a new molecular marker in human colorectal tumors. Cancer Res. 66:1949-1955. - PubMed
-
- Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. - PubMed
-
- Brass, A. L., I. C. Huang, Y. Benita, S. P. John, M. N. Krishnan, E. M. Feeley, B. J. Ryan, J. L. Weyer, L. van der Weyden, E. Fikrig, D. J. Adams, R. J. Xavier, M. Farzan, and S. J. Elledge. 2009. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 139:1243-1254. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

