Overlapping regulation of CenH3 localization and histone H3 turnover by CAF-1 and HIR proteins in Saccharomyces cerevisiae
- PMID: 20944015
- PMCID: PMC3018296
- DOI: 10.1534/genetics.110.123117
Overlapping regulation of CenH3 localization and histone H3 turnover by CAF-1 and HIR proteins in Saccharomyces cerevisiae
Abstract
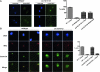
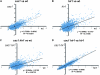
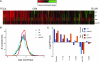
Accurate chromosome segregation is dependent on the centromere-specific histone H3 isoform known generally as CenH3, or as Cse4 in budding yeast. Cytological experiments have shown that Cse4 appears at extracentromeric loci in yeast cells deficient for both the CAF-1 and HIR histone H3/H4 deposition complexes, consistent with increased nondisjunction in these double mutant cells. Here, we examined molecular aspects of this Cse4 mislocalization. Genome-scale chromatin immunoprecipitation analyses demonstrated broader distribution of Cse4 outside of centromeres in cac1Δ hir1Δ double mutant cells that lack both CAF-1 and HIR complexes than in either single mutant. However, cytological localization showed that the essential inner kinetochore component Mif2 (CENP-C) was not recruited to extracentromeric Cse4 in cac1Δ hir1Δ double mutant cells. We also observed that rpb1-1 mutants displayed a modestly increased Cse4 half-life at nonpermissive temperatures, suggesting that turnover of Cse4 is partially dependent on Pol II transcription. We used genome-scale assays to demonstrate that the CAF-1 and HIR complexes independently stimulate replication-independent histone H3 turnover rates. We discuss ways in which altered histone exchange kinetics may affect eviction of Cse4 from noncentromeric loci.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

