Nonenzymatic free radical-catalyzed generation of 15-deoxy-Δ(12,14)-prostaglandin J₂-like compounds (deoxy-J₂-isoprostanes) in vivo
- PMID: 20944061
- PMCID: PMC2999919
- DOI: 10.1194/jlr.M010264
Nonenzymatic free radical-catalyzed generation of 15-deoxy-Δ(12,14)-prostaglandin J₂-like compounds (deoxy-J₂-isoprostanes) in vivo
Abstract
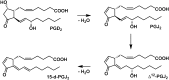
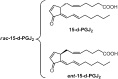
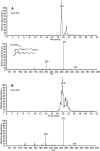
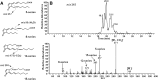
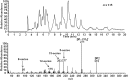
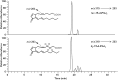
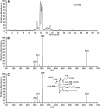
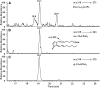
15-Deoxy-Δ(12,14)-prostaglandin J₂ (15-d-PGJ₂) is a reactive cyclopentenone eicosanoid generated from the dehydration of cyclooxygenase-derived prostaglandin D₂ (PGD₂). This compound possesses an α,β-unsaturated carbonyl moiety that can readily adduct thiol-containing biomolecules such as glutathione and cysteine residues of proteins via the Michael addition. Due to its reactivity, 15-d-PGJ₂ is thought to modulate inflammatory and apoptotic processes and is believed to be an endogenous ligand for peroxisome proliferator-activated receptor-γ. However, the extent to which 15-d-PGJ₂ is formed in vivo and the mechanisms that regulate its formation are unknown. Previously, we have reported the formation of PGD₂ and PGJ₂-like compounds, termed D₂/J₂-isoprostanes (D₂/J₂-IsoPs), produced in vivo by the free radical-catalyzed peroxidation of arachidonic acid (AA). Based on these findings, we investigated whether 15-d-PGJ₂-like compounds are also formed via this nonenzymatic pathway. Here we report the generation of novel 15-d-PGJ₂-like compounds, termed deoxy-J₂-isoprostanes (deoxy-J₂-IsoPs), in vivo, via the nonenzymatic peroxidation of AA. Levels of deoxy-J₂-IsoPs increased 12-fold (6.4 ± 1.1 ng/g liver) in rats after oxidant insult by CCl₄ treatment, compared with basal levels (0.55 ± 0.21 ng/g liver). These compounds may have important bioactivities in vivo under conditions associated with oxidant stress.
Figures

Similar articles
-
Comparison of formation of D2/E2-isoprostanes and F2-isoprostanes in vitro and in vivo--effects of oxygen tension and glutathione.Arch Biochem Biophys. 1998 May 1;353(1):160-71. doi: 10.1006/abbi.1998.0645. Arch Biochem Biophys. 1998. PMID: 9578611
-
Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid.J Biol Chem. 2008 May 2;283(18):12043-55. doi: 10.1074/jbc.M800122200. Epub 2008 Feb 10. J Biol Chem. 2008. PMID: 18263929 Free PMC article.
-
Formation of prostaglandins E2 and D2 via the isoprostane pathway: a mechanism for the generation of bioactive prostaglandins independent of cyclooxygenase.J Biol Chem. 2003 Aug 1;278(31):28479-89. doi: 10.1074/jbc.M303984200. Epub 2003 May 13. J Biol Chem. 2003. PMID: 12746435
-
15-Deoxy-Delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses.Chem Res Toxicol. 2008 Jan;21(1):138-44. doi: 10.1021/tx700177j. Epub 2007 Dec 4. Chem Res Toxicol. 2008. PMID: 18052108 Review.
-
15-deoxy-Delta12,14-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factors.Biochem Pharmacol. 2006 Nov 30;72(11):1516-28. doi: 10.1016/j.bcp.2006.07.030. Epub 2006 Sep 20. Biochem Pharmacol. 2006. PMID: 16987499 Review.
Cited by
-
Isoprostane generation and function.Chem Rev. 2011 Oct 12;111(10):5973-96. doi: 10.1021/cr200160h. Epub 2011 Aug 18. Chem Rev. 2011. PMID: 21848345 Free PMC article. Review. No abstract available.
-
The isoprostanes--25 years later.Biochim Biophys Acta. 2015 Apr;1851(4):433-45. doi: 10.1016/j.bbalip.2014.10.007. Epub 2014 Oct 30. Biochim Biophys Acta. 2015. PMID: 25449649 Free PMC article. Review.
-
Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids.Annu Rev Physiol. 2014;76:79-105. doi: 10.1146/annurev-physiol-021113-170341. Epub 2013 Oct 16. Annu Rev Physiol. 2014. PMID: 24161076 Free PMC article. Review.
-
Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases.Oxid Med Cell Longev. 2014;2014:572491. doi: 10.1155/2014/572491. Epub 2014 Apr 29. Oxid Med Cell Longev. 2014. PMID: 24868314 Free PMC article. Review.
-
Identification of a novel series of anti-inflammatory and anti-oxidative phospholipid oxidation products containing the cyclopentenone moiety in vitro and in vivo: Implication in atherosclerosis.J Biol Chem. 2017 Mar 31;292(13):5378-5391. doi: 10.1074/jbc.M116.751909. Epub 2017 Feb 15. J Biol Chem. 2017. PMID: 28202546 Free PMC article.
References
-
- Hardman J. G., Limbird L. E., 2001. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th edition McGraw-Hill Book Co, NY: 669–679.
-
- Marnett L. J. 2000. Cyclooxygenase mechanisms. Curr. Opin. Chem. Biol. 4: 545–552. - PubMed
-
- Marnett L. J., Rowlinson S. W., Goodwin D. C., Kalgutkar A. S., Lanzo C. A. 1999. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 274: 22903–22906. - PubMed
-
- Hamberg M., Samuelsson B. 1966. Prostaglandins in human seminal plasma. Prostaglandins and related factors 46. J. Biol. Chem. 241: 257–263. - PubMed
-
- Fitzpatrick F. A., Wynalda M. A. 1983. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J. Biol. Chem. 258: 11713–11718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

