Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein
- PMID: 20944063
- PMCID: PMC2973885
- DOI: 10.1073/pnas.1004380107
Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein
Abstract
Coxiella burnetii and Legionella pneumophila are evolutionarily related pathogens with different intracellular infection strategies. C. burnetii persists within and is transmitted by mammalian hosts, whereas, L. pneumophila is found primarily in the environment associated with protozoan hosts. Although a type IV secretion system encoded by the defect in organelle trafficking (dot) and intracellular multiplication (icm) genes is a virulence determinant that remains highly conserved in both bacteria, the two pathogens encode a different array of effector proteins that are delivered into host cells by the Dot/Icm machinery. This difference suggests that adaptations to evolutionarily distinct hosts may be reflected in the effector protein repertoires displayed by these two pathogens. Here we provide evidence in support of this hypothesis. We show that a unique C. burnetii effector from the ankyrin repeat (Ank) family called AnkG interferes with the mammalian apoptosis pathway. AnkG was found to interact with the host protein gC1qR (p32). Either the addition of AnkG to the repertoire of L. pneumophila effector proteins or the silencing of p32 in mouse dendritic cells resulted in a gain of function that allowed intracellular replication of L. pneumophila in these normally restrictive mammalian host cells by preventing rapid pathogen-induced apoptosis. These data indicate that p32 regulates pathogen-induced apoptosis and that AnkG functions to block this pathway. Thus, emergence of an effector protein that interferes with a proapoptotic signaling pathway directed against intracellular bacteria correlates with adaptation of a pathogen to mammalian hosts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
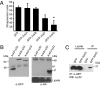
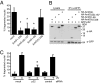
References
-
- Fields BS. The molecular ecology of Legionellae. Trends Microbiol. 1996;4:286–290. - PubMed
-
- Ninio S, Roy CR. Effector proteins translocated by Legionella pneumophila: Strength in numbers. Trends Microbiol. 2007;15:372–380. - PubMed
-
- Fraser DW, et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

