Deconstructing thermodynamic parameters of a coupled system from site-specific observables
- PMID: 20944067
- PMCID: PMC2973918
- DOI: 10.1073/pnas.1003609107
Deconstructing thermodynamic parameters of a coupled system from site-specific observables
Abstract
Cooperative interactions mediate information transfer between structural domains of a protein molecule and are major determinants of protein function and modulation. The prevalent theories to understand the thermodynamic origins of cooperativity have been developed to reproduce the complex behavior of a global thermodynamic observable such as ligand binding or enzyme activity. However, in most cases the measurement of a single global observable cannot uniquely define all the terms that fully describe the energetics of the system. Here we establish a theoretical groundwork for analyzing protein thermodynamics using site-specific information. Our treatment involves extracting a site-specific parameter (defined as χ value) associated with a structural unit. We demonstrate that, under limiting conditions, the χ value is related to the direct interaction terms associated with the structural unit under observation and its intrinsic activation energy. We also introduce a site-specific interaction energy term (χ(diff)) that is a function of the direct interaction energy of that site with every other site in the system. When combined with site-directed mutagenesis and other molecular level perturbations, analyses of χ values of site-specific observables may provide valuable insights into protein thermodynamics and structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
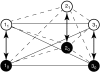

 (C), and 5,
(C), and 5,  (E), were varied over 6 orders of magnitude:
(E), were varied over 6 orders of magnitude:  (∘), 0 (+), 3(▪) (i = 1 or 5). Interaction between resting states of particles 1 and 5, θ1050 (D), and that between resting states of particles 2 and 5, θ2050 (F), were varied over 6 orders of magnitude: log θi050 = -3 (∘), 0 (+), 3(▪) (i = 1 or 2). Vertical dashed line corresponds to the voltage axis at 0 mV. Extrapolating the linear segments of the traces (at high and low voltages) to this axis gives the corresponding χ values.
(∘), 0 (+), 3(▪) (i = 1 or 5). Interaction between resting states of particles 1 and 5, θ1050 (D), and that between resting states of particles 2 and 5, θ2050 (F), were varied over 6 orders of magnitude: log θi050 = -3 (∘), 0 (+), 3(▪) (i = 1 or 2). Vertical dashed line corresponds to the voltage axis at 0 mV. Extrapolating the linear segments of the traces (at high and low voltages) to this axis gives the corresponding χ values.Similar articles
-
Thermodynamics of information transfer between subunits in oligomeric enzymes and kinetic cooperativity. 2. Thermodynamics of kinetic cooperativity.Eur J Biochem. 1990 Dec 12;194(2):475-81. doi: 10.1111/j.1432-1033.1990.tb15641.x. Eur J Biochem. 1990. PMID: 2269279
-
The determination of thermodynamic allosteric parameters of an enzyme undergoing steady-state turnover.Arch Biochem Biophys. 1983 Jul 1;224(1):389-401. doi: 10.1016/0003-9861(83)90225-4. Arch Biochem Biophys. 1983. PMID: 6870263
-
Thermodynamic model of cooperativity in a dimeric protein: unique and independent parameters formulation.Biophys Chem. 1992 Dec;45(2):181-91. doi: 10.1016/0301-4622(92)87010-g. Biophys Chem. 1992. PMID: 1286151
-
Cooperativity in monomeric enzymes with single ligand-binding sites.Bioorg Chem. 2012 Aug;43:44-50. doi: 10.1016/j.bioorg.2011.11.001. Epub 2011 Nov 17. Bioorg Chem. 2012. PMID: 22137502 Free PMC article. Review.
-
Thermodynamics of protein-ligand interactions: history, presence, and future aspects.J Recept Signal Transduct Res. 2004 Feb;24(1-2):1-52. doi: 10.1081/rrs-120037896. J Recept Signal Transduct Res. 2004. PMID: 15344878 Review.
Cited by
-
The contribution of voltage clamp fluorometry to the understanding of channel and transporter mechanisms.J Gen Physiol. 2019 Oct 7;151(10):1163-1172. doi: 10.1085/jgp.201912372. Epub 2019 Aug 20. J Gen Physiol. 2019. PMID: 31431491 Free PMC article. Review.
-
Perspectives on: conformational coupling in ion channels: thermodynamics of electromechanical coupling in voltage-gated ion channels.J Gen Physiol. 2012 Dec;140(6):613-23. doi: 10.1085/jgp.201210840. J Gen Physiol. 2012. PMID: 23183697 Free PMC article. No abstract available.
-
Free-energy relationships in ion channels activated by voltage and ligand.J Gen Physiol. 2013 Jan;141(1):11-28. doi: 10.1085/jgp.201210860. Epub 2012 Dec 17. J Gen Physiol. 2013. PMID: 23250866 Free PMC article.
-
Linkage analysis reveals allosteric coupling in Kir2.1 channels.J Gen Physiol. 2018 Nov 5;150(11):1541-1553. doi: 10.1085/jgp.201812127. Epub 2018 Oct 16. J Gen Physiol. 2018. PMID: 30327330 Free PMC article.
-
Perspectives on: conformational coupling in ion channels: conformational coupling in BK potassium channels.J Gen Physiol. 2012 Dec;140(6):625-34. doi: 10.1085/jgp.201210849. J Gen Physiol. 2012. PMID: 23183698 Free PMC article.
References
-
- Monod J, Jacob F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Sym Quant Biol. 1961;26:389–401. - PubMed
-
- Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228(5273):726–739. - PubMed
-
- Perutz MF, Wilkinson AJ, Paoli M, Dodson GG. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu Rev Biophys Biomol Struct. 1998;27:1–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

