Langerhans cells are not required for graft-versus-host disease
- PMID: 20944073
- PMCID: PMC3031488
- DOI: 10.1182/blood-2010-07-299073
Langerhans cells are not required for graft-versus-host disease
Abstract
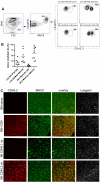
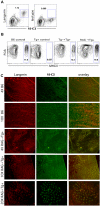
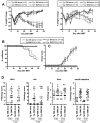
Graft-versus-host disease (GVHD) is initiated and maintained by antigen-presenting cells (APCs) that prime alloreactive donor T cells. APCs are therefore attractive targets for GVHD prevention and treatment. APCs are diverse in phenotype and function, making understanding how APC subsets contribute to GVHD necessary for the development of APC-targeted therapies. Langerhans cells (LCs) have been shown to be sufficient to initiate skin GVHD in a major histocompatibility complex-mismatched model; however, their role when other host APC subsets are intact is unknown. To address this question, we used mice genetically engineered to be deficient in LCs by virtue of expression of diphtheria toxin A under the control of a BAC (bacterial artificial chromosome) transgenic hu-man Langerin locus. Neither CD8- nor CD4-mediated GVHD was diminished in recipients lacking LCs. Similarly, CD8- and CD4-mediated GVHD, including that in the skin, was unaffected if bone marrow came from donors that could not generate LCs, even though donor LCs engrafted in control mice. Engraftment of donor LCs after irradiation in wild-type hosts required donor T cells, with immunofluorescence revealing patches of donor and residual host LCs. Surprisingly, donor LC engraftment in Langerin-diphtheria toxin A (DTA) transgenic hosts was independent of donor T cells, suggesting that a Langerin(+) cell regulates repopulation of the LC compartment.
Figures




Similar articles
-
Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis.J Invest Dermatol. 2009 Dec;129(12):2805-17. doi: 10.1038/jid.2009.176. Epub 2009 Jun 25. J Invest Dermatol. 2009. PMID: 19554018 Free PMC article.
-
Numerical, morphological and phenotypic changes in Langerhans cells in the course of murine graft-versus-host disease.Br J Dermatol. 2001 Dec;145(6):918-27. doi: 10.1046/j.1365-2133.2001.04539.x. Br J Dermatol. 2001. PMID: 11899145
-
Acute ablation of Langerhans cells enhances skin immune responses.J Immunol. 2010 Oct 15;185(8):4724-8. doi: 10.4049/jimmunol.1001802. Epub 2010 Sep 20. J Immunol. 2010. PMID: 20855870 Free PMC article.
-
A crucial role for host APCs in the induction of donor CD4+CD25+ regulatory T cell-mediated suppression of experimental graft-versus-host disease.J Immunol. 2010 Oct 1;185(7):3866-72. doi: 10.4049/jimmunol.1001625. Epub 2010 Sep 1. J Immunol. 2010. PMID: 20810991 Free PMC article.
-
Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):48-54. doi: 10.1016/j.bcmd.2007.06.019. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827036 Review.
Cited by
-
A question of persistence: Langerhans cells and graft-versus-host disease.Exp Dermatol. 2014 Apr;23(4):234-5. doi: 10.1111/exd.12325. Exp Dermatol. 2014. PMID: 24443966 Free PMC article.
-
Induction of acute GVHD by sex-mismatched H-Y antigens in the absence of functional radiosensitive host hematopoietic-derived antigen-presenting cells.Blood. 2012 Apr 19;119(16):3844-53. doi: 10.1182/blood-2011-10-384057. Epub 2011 Nov 18. Blood. 2012. PMID: 22101894 Free PMC article.
-
Redefining the Role of Langerhans Cells As Immune Regulators within the Skin.Front Immunol. 2018 Jan 5;8:1941. doi: 10.3389/fimmu.2017.01941. eCollection 2017. Front Immunol. 2018. PMID: 29379502 Free PMC article. Review.
-
Langerhans cells regulate cutaneous injury by licensing CD8 effector cells recruited to the skin.Blood. 2011 Jun 30;117(26):7063-9. doi: 10.1182/blood-2011-01-329185. Epub 2011 May 12. Blood. 2011. PMID: 21566096 Free PMC article.
-
The intimate relationship between human cytomegalovirus and the dendritic cell lineage.Front Microbiol. 2014 Aug 7;5:389. doi: 10.3389/fmicb.2014.00389. eCollection 2014. Front Microbiol. 2014. PMID: 25147545 Free PMC article. Review.
References
-
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352. - PubMed
-
- Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8(6):575–581. - PubMed
-
- Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172(12):7393–7398. - PubMed
-
- Koyama M, Hashimoto D, Aoyama K, et al. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113(9):2088–2095. - PubMed
-
- Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10(9):987–992. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

