A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis
- PMID: 20944089
- PMCID: PMC3110937
- DOI: 10.1126/scitranslmed.3001094
A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis
Abstract
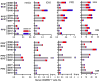
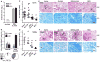
Despite the widespread use of the childhood vaccine against tuberculosis (TB), Mycobacterium bovis bacillus Calmette-Guérin (BCG), the disease remains a serious global health problem. A successful vaccine against TB that replaces or boosts BCG would include antigens that induce or recall the appropriate T cell responses. Four Mycobacterium tuberculosis (Mtb) antigens--including members of the virulence factor families PE/PPE and EsX or antigens associated with latency--were produced as a single recombinant fusion protein (ID93). When administered together with the adjuvant GLA-SE, a stable oil-in-water nanoemulsion, the fusion protein was immunogenic in mice, guinea pigs, and cynomolgus monkeys. In mice, this fusion protein-adjuvant combination induced polyfunctional CD4 T helper 1 cell responses characterized by antigen-specific interferon-γ, tumor necrosis factor, and interleukin-2, as well as a reduction in the number of bacteria in the lungs of animals after they were subsequently infected with virulent or multidrug-resistant Mtb strains. Furthermore, boosting BCG-vaccinated guinea pigs with fusion peptide-adjuvant resulted in reduced pathology and fewer bacilli, and prevented the death of animals challenged with virulent Mtb. Finally, the fusion protein elicited polyfunctional effector CD4 and CD8 T cell responses in BCG-vaccinated or Mtb-exposed human peripheral blood mononuclear cells. This study establishes that the protein subunit vaccine consisting of the fusion protein and adjuvant protects against TB and drug-resistant TB in animals and is a candidate for boosting the protective efficacy of the childhood BCG vaccine in humans.
Conflict of interest statement
Competing interests: The authors have no competing interests.
Figures




References
-
- WHO. Fourth global report. World Health Organization; 2008. Anti-tuberculosis drug resistance in the world.
-
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. - PubMed
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, Chesko J, Coler RN, Friede M, Reed SG, Vedvick TS. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75:123–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

