Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease
- PMID: 20944662
- PMCID: PMC3124757
- DOI: 10.1038/nrn2915
Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease
Abstract
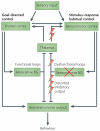
Progressive loss of the ascending dopaminergic projection in the basal ganglia is a fundamental pathological feature of Parkinson's disease. Studies in animals and humans have identified spatially segregated functional territories in the basal ganglia for the control of goal-directed and habitual actions. In patients with Parkinson's disease the loss of dopamine is predominantly in the posterior putamen, a region of the basal ganglia associated with the control of habitual behaviour. These patients may therefore be forced into a progressive reliance on the goal-directed mode of action control that is mediated by comparatively preserved processing in the rostromedial striatum. Thus, many of their behavioural difficulties may reflect a loss of normal automatic control owing to distorting output signals from habitual control circuits, which impede the expression of goal-directed action.
Figures

References
-
- Ferrier D. The Functions of the Brain. Putnam’s Sons; New York: 1876.
-
- Penney JB, Jr, Young AB. Striatal inhomogeneities and basal ganglia function. Mov. Disord. 1986;1:3–15. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
-
Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–281. This review introduced a conceptual development in suggesting that a pause in neuronal firing in basal ganglia output nuclei disinhibits efferent targets and is the major physiological mechanism by which the basal ganglia exert their effects on behaviour.
-
-
- Gerfen CR, Wilson CJ. In: Handbook of Chemical Neuroanatomy. Swanson LW, Bjorklund A, Hokfelt T, editors. Vol 12. Elsevier; Amsterdam: 1996. pp. 371–468.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

