Cerebrolysin adjuvant treatment in Broca's aphasics following first acute ischemic stroke of the left middle cerebral artery
- PMID: 20945821
- PMCID: PMC3019000
Cerebrolysin adjuvant treatment in Broca's aphasics following first acute ischemic stroke of the left middle cerebral artery
Erratum in
- J Med Life. 2011 Jan-Mar;4(1):7 p following 123. Petria, Ligia [corrected to Petrica, Ligia]
Abstract
Background: The aim of our study was to assess the efficacy of Cerebrolysin administration in Broca's aphasics with acute ischemic stroke.
Methods: We registered 2212 consecutive Broca's aphasics following an acute ischemic stroke admitted in four departments of neurology in Romania, between September 2005 and September 2009. Language was evaluated with the Romanian version of the Western Aphasia Battery (WAB). The following inclusion criteria were used for this study: age 20-75 years, admission in the hospital within 12 hours from the onset of the symptoms, diagnosis of first acute left middle cerebral artery (MCA) ischemic stroke, presence of large artery disease (LAD) stroke, a NIHSS score of 5-22 points, and a therapeutic time window within 72 h. Fifty two patients were treated with Cerebrolysin (Cerebrolysin group) as an adjunctive treatment. A placebo group, which received saline infusions (n=104 patients) were matched to the NIHSS and WAB scores, gender and age of the Cerebrolysin group at baseline. We assessed spontaneous speech (SS), comprehension (C), repetition (R), naming (N), and Aphasia Quotient (AQ) scores of the two groups in an open label design, over 90 days, the mRS scores and mortality.
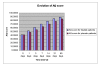
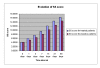
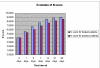
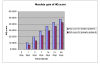
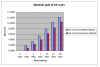
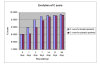
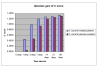
Results: The Cerebrolysin and the placebo groups had similar age (66 +/- 8 versus 65 +/- 8 years) and sex ratio (14/38 versus 30/74). The mean AQ scores and the mean subscores for 3 subtests of WAB (SS, R, N) were similar at baseline and improved in the Cerebrolysin group significantly (p < 0.05) over placebo group at all study time points. The mRS score at 90 days was also lower in the Cerebrolysin group than in the placebo group. Cerebrolysin and placebo were both tolerated and safe, and no difference in the mortality rate was seen (3.8% in each group).
Conclusion: Cerebrolysin is effective for the treatment of Broca's aphasics with a first acute ischemic stroke of the left MCA territory.
Figures





References
-
- Barolin GS, Koppi S. Old and new aspects of stroke treatment with emphasis on metabolically active medication and rehabilitative outcome . Eurorehab. 1996;31:135–143.
-
- Basso A, Lecours AR. Anatomoclinical correlations of the aphasias as defined through computerized tomography . Brain Lang. 1985;26:201–229. - PubMed
-
- Broderick JP, Hacke W. Treatment of Acute Ischemic Stroke . Neuroprotection and Medical Management % Circulation. 2002;106:1736–1740. - PubMed
-
- DeKeyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischemic stroke . Trends Neurosci. 1999;22:535–540. - PubMed
-
- Ferro JM, Davalos A. Other neuroprotective therapies on trial in acute stroke . Cerebrovasc Dis . 2006;21:127–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
