Design, synthesis, crystal structures, and antimicrobial activity of sulfonamide boronic acids as β-lactamase inhibitors
- PMID: 20945905
- PMCID: PMC3166525
- DOI: 10.1021/jm101015z
Design, synthesis, crystal structures, and antimicrobial activity of sulfonamide boronic acids as β-lactamase inhibitors
Abstract
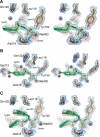
We investigated a series of sulfonamide boronic acids that resulted from the merging of two unrelated AmpC β-lactamase inhibitor series. The new boronic acids differed in the replacement of the canonical carboxamide, found in all penicillin and cephalosporin antibiotics, with a sulfonamide. Surprisingly, these sulfonamides had a highly distinct structure-activity relationship from the previously explored carboxamides, high ligand efficiencies (up to 0.91), and K(i) values down to 25 nM and up to 23 times better for smaller analogues. Conversely, K(i) values were 10-20 times worse for larger molecules than in the carboxamide congener series. X-ray crystal structures (1.6-1.8 Å) of AmpC with three of the new sulfonamides suggest that this altered structure-activity relationship results from the different geometry and polarity of the sulfonamide versus the carboxamide. The most potent inhibitor reversed β-lactamase-mediated resistance to third generation cephalosporins, lowering their minimum inhibitory concentrations up to 32-fold in cell culture.
Figures

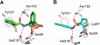



Similar articles
-
The complexed structure and antimicrobial activity of a non-beta-lactam inhibitor of AmpC beta-lactamase.Protein Sci. 1999 Nov;8(11):2330-7. doi: 10.1110/ps.8.11.2330. Protein Sci. 1999. PMID: 10595535 Free PMC article.
-
Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase.J Med Chem. 1998 Nov 5;41(23):4577-86. doi: 10.1021/jm980343w. J Med Chem. 1998. PMID: 9804697
-
Nanomolar inhibitors of AmpC beta-lactamase.J Am Chem Soc. 2003 Jan 22;125(3):685-95. doi: 10.1021/ja0288338. J Am Chem Soc. 2003. PMID: 12526668
-
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations.Drugs. 2018 Jan;78(1):65-98. doi: 10.1007/s40265-017-0851-9. Drugs. 2018. PMID: 29230684 Review.
-
Resistance to beta-lactam antibiotics: structure and mechanism based design of beta-lactamase inhibitors.Curr Med Chem. 2002 Jun;9(12):1145-65. doi: 10.2174/0929867023370031. Curr Med Chem. 2002. PMID: 12052169 Review.
Cited by
-
Fragment-guided design of subnanomolar β-lactamase inhibitors active in vivo.Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17448-53. doi: 10.1073/pnas.1208337109. Epub 2012 Oct 5. Proc Natl Acad Sci U S A. 2012. PMID: 23043117 Free PMC article.
-
Crystal Structures of KPC-2 and SHV-1 β-Lactamases in Complex with the Boronic Acid Transition State Analog S02030.Antimicrob Agents Chemother. 2016 Jan 4;60(3):1760-6. doi: 10.1128/AAC.02643-15. Antimicrob Agents Chemother. 2016. PMID: 26729491 Free PMC article.
-
Discovery of Boronic Acids-Based β-Lactamase Inhibitors Through In Situ Click Chemistry.Int J Mol Sci. 2025 Apr 28;26(9):4182. doi: 10.3390/ijms26094182. Int J Mol Sci. 2025. PMID: 40362418 Free PMC article.
-
The impact of library size and scale of testing on virtual screening.Nat Chem Biol. 2025 Jul;21(7):1039-1045. doi: 10.1038/s41589-024-01797-w. Epub 2025 Jan 3. Nat Chem Biol. 2025. PMID: 39753705
-
One ring to rule them all: Current trends in combating bacterial resistance to the β-lactams.Protein Sci. 2016 Apr;25(4):787-803. doi: 10.1002/pro.2889. Epub 2016 Mar 9. Protein Sci. 2016. PMID: 26813250 Free PMC article. Review.
References
-
- Livermore DM. beta-Lactamases- the Threat Renews. Curr. Protein Pept. Sci. 2009;10:397–400. - PubMed
-
- Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 2005;105:395–424. - PubMed
-
- Wilke MS, Lovering AL, Strynadka NC. Beta-lactam antibiotic resistance: a current structural perspective. Curr. Opin. Microbiol. 2005;8:525–533. - PubMed
-
- Lode HM. Rational antibiotic therapy and the position of ampicillin/sulbactam. Int. J. Antimicrob. Agents. 2008;32:10–28. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

