17-allyamino-17-demethoxygeldanamycin treatment results in a magnetic resonance spectroscopy-detectable elevation in choline-containing metabolites associated with increased expression of choline transporter SLC44A1 and phospholipase A2
- PMID: 20946630
- PMCID: PMC3096977
- DOI: 10.1186/bcr2729
17-allyamino-17-demethoxygeldanamycin treatment results in a magnetic resonance spectroscopy-detectable elevation in choline-containing metabolites associated with increased expression of choline transporter SLC44A1 and phospholipase A2
Abstract
Introduction: 17-allyamino-17-demethoxygeldanamycin (17-AAG), a small molecule inhibitor of Hsp90, is currently in clinical trials in breast cancer. However, 17-AAG treatment often results in inhibition of tumor growth rather than shrinkage, making detection of response a challenge. Magnetic resonance spectroscopy (MRS) and spectroscopic imaging (MRSI) are noninvasive imaging methods than can be used to monitor metabolic biomarkers of drug-target modulation. This study set out to examine the MRS-detectable metabolic consequences of Hsp90 inhibition in a breast cancer model.
Methods: MCF-7 breast cancer cells were investigated, and MRS studies were performed both on live cells and on cell extracts. (31)P and (1)H MRS were used to determine total cellular metabolite concentrations and (13)C MRS was used to probe the metabolism of [1,2-(13)C]-choline. To explain the MRS metabolic findings, microarray and RT-PCR were used to analyze gene expression, and in vitro activity assays were performed to determine changes in enzymatic activity following 17-AAG treatment.
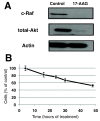
Results: Treatment of MCF-7 cells with 17-AAG for 48 hours caused a significant increase in intracellular levels of choline (to 266 ± 18% of control, P = 0.05) and phosphocholine (PC; to 181 ± 10% of control, P = 0.001) associated with an increase in expression of choline transporter SLC44A1 and an elevation in the de novo synthesis of PC. We also detected an increase in intracellular levels of glycerophosphocholine (GPC; to 176 ± 38% of control, P = 0.03) associated with an increase in PLA2 expression and activity.
Conclusions: This study determined that in the MCF-7 breast cancer model inhibition of Hsp90 by 17-AAG results in a significant MRS-detectable increase in choline, PC and GPC, which is likely due to an increase in choline transport into the cell and phospholipase activation. (1)H MRSI can be used in the clinical setting to detect levels of total choline-containing metabolite (t-Cho, composed of intracellular choline, PC and GPC). As Hsp90 inhibitors enter routine clinical use, t-Cho could thus provide an easily detectable, noninvasive metabolic biomarker of Hsp90 inhibition in breast cancer patients.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

