Krüppel-like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation
- PMID: 20946687
- PMCID: PMC2965135
- DOI: 10.1186/1742-2094-7-68
Krüppel-like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation
Abstract
Background: Activation of microglia, the resident macrophages of the central nervous system (CNS), is the hallmark of neuroinflammation in neurodegenerative diseases and other pathological conditions associated with CNS infection. The activation of microglia is often associated with bystander neuronal death. Nuclear factor-κB (NF-κB) is one of the important transcription factors known to be associated with microglial activation which upregulates the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (Cox-2) and other pro-inflammatory cytokines. Recent studies have focused on the role of Krüppel-like factor 4 (Klf4), one of the zinc-finger transcription factors, in mediating inflammation. However, these studies were limited to peripheral system and its role in CNS is not understood. Our studies focused on the possible role of Klf4 in mediating CNS inflammation.
Methods: For in vitro studies, mouse microglial BV-2 cell lines were treated with 500 ng/ml Salmonella enterica lipopolysacchride (LPS). Brain tissues were isolated from BALB/c mice administered with 5 mg/kg body weight of LPS. Expressions of Klf4, Cox-2, iNOS and pNF-κB were evaluated using western blotting, quantitative real time PCR, and reverse transcriptase polymerase chain reactions (RT-PCRs). Klf4 knockdown was carried out using SiRNA specific for Klf4 mRNA and luciferase assays and electromobility shift assay (EMSA) were performed to study the interaction of Klf4 to iNOS promoter elements in vitro. Co-immunoprecipitation of Klf4 and pNF-κB was done in order to study a possible interaction between the two transcription factors.
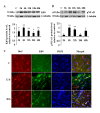
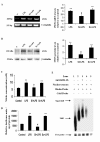
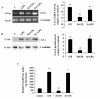
Results: LPS stimulation increased Klf4 expression in microglial cells in a time- and dose-dependent manner. Knockdown of Klf4 resulted in decreased levels of the pro-inflammatory cytokines TNF-α, MCP-1 and IL-6, along with a significant decrease in iNOS and Cox-2 expression. NO production also decreased as a result of Klf4 knockdown. We found that Klf4 can potentially interact with pNF-κB and is important for iNOS and Cox-2 promoter activity in vitro.
Conclusions: These studies demonstrate the role of Klf4 in microglia in mediating neuroinflammation in response to the bacterial endotoxin LPS.
Figures




Similar articles
-
Therapeutic targeting of Krüppel-like factor 4 abrogates microglial activation.J Neuroinflammation. 2012 Mar 19;9:57. doi: 10.1186/1742-2094-9-57. J Neuroinflammation. 2012. PMID: 22429472 Free PMC article.
-
YC-1 attenuates LPS-induced proinflammatory responses and activation of nuclear factor-kappaB in microglia.Br J Pharmacol. 2007 Jun;151(3):396-405. doi: 10.1038/sj.bjp.0707187. Epub 2007 Apr 2. Br J Pharmacol. 2007. PMID: 17401444 Free PMC article.
-
Inhibitory effect of a tyrosine-fructose Maillard reaction product, 2,4-bis(p-hydroxyphenyl)-2-butenal on amyloid-β generation and inflammatory reactions via inhibition of NF-κB and STAT3 activation in cultured astrocytes and microglial BV-2 cells.J Neuroinflammation. 2011 Oct 7;8:132. doi: 10.1186/1742-2094-8-132. J Neuroinflammation. 2011. PMID: 21982455 Free PMC article.
-
A review of KLF4 and inflammatory disease: Current status and future perspective.Pharmacol Res. 2024 Sep;207:107345. doi: 10.1016/j.phrs.2024.107345. Epub 2024 Aug 10. Pharmacol Res. 2024. PMID: 39134187 Review.
-
Is cyclooxygenase-1 involved in neuroinflammation?J Neurosci Res. 2021 Nov;99(11):2976-2998. doi: 10.1002/jnr.24934. Epub 2021 Aug 4. J Neurosci Res. 2021. PMID: 34346520 Free PMC article. Review.
Cited by
-
Krüpple-like factors in the central nervous system: novel mediators in stroke.Metab Brain Dis. 2015 Apr;30(2):401-10. doi: 10.1007/s11011-013-9468-1. Epub 2013 Dec 15. Metab Brain Dis. 2015. PMID: 24338065 Free PMC article. Review.
-
Whole-transcriptome sequencing identifies neuroinflammation, metabolism and blood-brain barrier related processes in the hippocampus of aged mice during perioperative period.CNS Neurosci Ther. 2022 Oct;28(10):1576-1595. doi: 10.1111/cns.13901. Epub 2022 Jul 27. CNS Neurosci Ther. 2022. PMID: 35899365 Free PMC article.
-
RIG-I mediates innate immune response in mouse neurons following Japanese encephalitis virus infection.PLoS One. 2011;6(6):e21761. doi: 10.1371/journal.pone.0021761. Epub 2011 Jun 30. PLoS One. 2011. PMID: 21738791 Free PMC article.
-
Molecular hallmarks of excitatory and inhibitory neuronal resilience and resistance to Alzheimer's disease.bioRxiv [Preprint]. 2025 Jan 15:2025.01.13.632801. doi: 10.1101/2025.01.13.632801. bioRxiv. 2025. PMID: 39868232 Free PMC article. Preprint.
-
MicroRNAs in the brain: it's regulatory role in neuroinflammation.Mol Neurobiol. 2013 Jun;47(3):1034-44. doi: 10.1007/s12035-013-8400-3. Epub 2013 Jan 12. Mol Neurobiol. 2013. PMID: 23315269 Review.
References
-
- Beyer M, Gimsa U, Eyupoglu IY, Hailer NP, Nitsch R. Phagocytosis of neuronal or glial debris by microglial cells: upregulation of MHC class II expression and multinuclear giant cell formation in vitro. Glia. 2000;31:262–6. doi: 10.1002/1098-1136(200009)31:3<262::AID-GLIA70>3.0.CO;2-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

