Temporal lobe epilepsy induces intrinsic alterations in Na channel gating in layer II medial entorhinal cortex neurons
- PMID: 20946956
- PMCID: PMC3014455
- DOI: 10.1016/j.nbd.2010.10.004
Temporal lobe epilepsy induces intrinsic alterations in Na channel gating in layer II medial entorhinal cortex neurons
Abstract
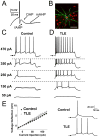
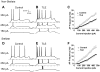
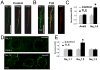
Temporal lobe epilepsy (TLE) is the most common form of adult epilepsy involving the limbic structures of the temporal lobe. Layer II neurons of the entorhinal cortex (EC) form the major excitatory input into the hippocampus via the perforant path and consist of non-stellate and stellate neurons. These neurons are spared and hyper-excitable in TLE. The basis for the hyper-excitability is likely multifactorial and may include alterations in intrinsic properties. In a rat model of TLE, medial EC (mEC) non-stellate and stellate neurons had significantly higher action potential (AP) firing frequencies than in control. The increase remained in the presence of synaptic blockers, suggesting intrinsic mechanisms. Since sodium (Na) channels play a critical role in AP generation and conduction we sought to determine if Na channel gating parameters and expression levels were altered in TLE. Na channel currents recorded from isolated mEC TLE neurons revealed increased Na channel conductances, depolarizing shifts in inactivation parameters and larger persistent (I(NaP)) and resurgent (I(NaR)) Na currents. Immunofluorescence experiments revealed increased staining of Na(v)1.6 within the axon initial segment and Na(v)1.2 within the cell bodies of mEC TLE neurons. These studies provide support for additional intrinsic alterations within mEC layer II neurons in TLE and implicate alterations in Na channel activity and expression, in part, for establishing the profound increase in intrinsic membrane excitability of mEC layer II neurons in TLE. These intrinsic changes, together with changes in the synaptic network, could support seizure activity in TLE.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



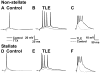




References
-
- Agrawal N, Alonso A, Ragsdale DS. Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia. 2003;44:1601–1604. - PubMed
-
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. Journal of Neurophysiology. 1993;70:128–143. - PubMed
-
- Apergis-Schoute J, Pinto A, Pare D. Ultrastructural organization of medial prefrontal inputs to the rhinal cortices. Eur J Neurosci. 2006;24:135–144. - PubMed
-
- Aronica E, Yankaya B, Troost D, van Vliet EA, Lopes da Silva FH, Gorter JA. Induction of neonatal sodium channel II and III alpha-isoform mRNAs in neurons and microglia after status epilepticus in the rat hippocampus. European Journal of Neuroscience. 2001;13:1261–1266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

