Novel channel enzyme fusion proteins confer arsenate resistance
- PMID: 20947511
- PMCID: PMC3000990
- DOI: 10.1074/jbc.M110.184457
Novel channel enzyme fusion proteins confer arsenate resistance
Abstract
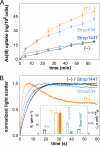
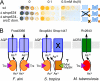
Steady exposure to environmental arsenic has led to the evolution of vital cellular detoxification mechanisms. Under aerobic conditions, a two-step process appears most common among microorganisms involving reduction of predominant, oxidized arsenate (H(2)As(V)O(4)(-)/HAs(V)O(4)(2-)) to arsenite (As(III)(OH)(3)) by a cytosolic enzyme (ArsC; Escherichia coli type arsenate reductase) and subsequent extrusion via ArsB (E. coli type arsenite transporter)/ACR3 (yeast type arsenite transporter). Here, we describe novel fusion proteins consisting of an aquaglyceroporin-derived arsenite channel with a C-terminal arsenate reductase domain of phosphotyrosine-phosphatase origin, providing transposable, single gene-encoded arsenate resistance. The fusion occurred in actinobacteria from soil, Frankia alni, and marine environments, Salinispora tropica; Mycobacterium tuberculosis encodes an analogous ACR3-ArsC fusion. Mutations rendered the aquaglyceroporin channel more polar resulting in lower glycerol permeability and enhanced arsenite selectivity. The arsenate reductase domain couples to thioredoxin and can complement arsenate-sensitive yeast strains. A second isoform with a nonfunctional channel may use the mycothiol/mycoredoxin cofactor pool. These channel enzymes constitute prototypes of a novel concept in metabolism in which a substrate is generated and compartmentalized by the same molecule. Immediate diffusion maintains the dynamic equilibrium and prevents toxic accumulation of metabolites in an energy-saving fashion.
Figures



Similar articles
-
Functional analysis of a chromosomal arsenic resistance operon in Pseudomonas fluorescens strain MSP3.Mol Biol Rep. 2001;28(2):63-72. doi: 10.1023/a:1017950207981. Mol Biol Rep. 2001. PMID: 11931390
-
Arsenate reductases in prokaryotes and eukaryotes.Environ Health Perspect. 2002 Oct;110 Suppl 5(Suppl 5):745-8. doi: 10.1289/ehp.02110s5745. Environ Health Perspect. 2002. PMID: 12426124 Free PMC article.
-
Analysis of genes involved in arsenic resistance in Corynebacterium glutamicum ATCC 13032.Appl Environ Microbiol. 2005 Oct;71(10):6206-15. doi: 10.1128/AEM.71.10.6206-6215.2005. Appl Environ Microbiol. 2005. PMID: 16204540 Free PMC article.
-
[Bacterial resistance to arsenic compounds].Rev Latinoam Microbiol. 1995 Oct-Dec;37(4):387-95. Rev Latinoam Microbiol. 1995. PMID: 8900573 Review. Spanish.
-
Arsenate reduction: thiol cascade chemistry with convergent evolution.J Mol Biol. 2006 Sep 8;362(1):1-17. doi: 10.1016/j.jmb.2006.07.002. Epub 2006 Aug 14. J Mol Biol. 2006. PMID: 16905151 Review.
Cited by
-
Identification and Genome Analysis of an Arsenic-Metabolizing Strain of Citrobacter youngae IITK SM2 in Middle Indo-Gangetic Plain Groundwater.Biomed Res Int. 2022 Mar 10;2022:6384742. doi: 10.1155/2022/6384742. eCollection 2022. Biomed Res Int. 2022. PMID: 35309170 Free PMC article.
-
Is Arsenic Exposure a Risk Factor for Metabolic Syndrome? A Review of the Potential Mechanisms.Front Endocrinol (Lausanne). 2022 May 16;13:878280. doi: 10.3389/fendo.2022.878280. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35651975 Free PMC article. Review.
-
Identification of Resistance Genes and Response to Arsenic in Rhodococcus aetherivorans BCP1.Front Microbiol. 2019 May 7;10:888. doi: 10.3389/fmicb.2019.00888. eCollection 2019. Front Microbiol. 2019. PMID: 31133997 Free PMC article.
-
Targeting Channels and Transporters in Protozoan Parasite Infections.Front Chem. 2018 Mar 27;6:88. doi: 10.3389/fchem.2018.00088. eCollection 2018. Front Chem. 2018. PMID: 29637069 Free PMC article. Review.
-
Aquaporins with anion/monocarboxylate permeability: mechanisms, relevance for pathogen-host interactions.Front Pharmacol. 2014 Sep 1;5:199. doi: 10.3389/fphar.2014.00199. eCollection 2014. Front Pharmacol. 2014. PMID: 25225485 Free PMC article. Review.
References
-
- Oremland R. S., Stolz J. F. (2003) Science 300, 939–944 - PubMed
-
- Turner A. W. (1949) Nature 164, 76–77 - PubMed
-
- Luecke H., Quiocho F. A. (1990) Nature 347, 402–406 - PubMed
-
- Moore S. A., Moennich D. M., Gresser M. J. (1983) J. Biol. Chem. 258, 6266–6271 - PubMed
-
- Messens J., Silver S. (2006) J. Mol. Biol. 362, 1–17 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

