Whole-body physiologically based pharmacokinetic model for nutlin-3a in mice after intravenous and oral administration
- PMID: 20947617
- PMCID: PMC3014266
- DOI: 10.1124/dmd.110.035915
Whole-body physiologically based pharmacokinetic model for nutlin-3a in mice after intravenous and oral administration
Abstract
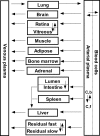
Nutlin-3a is an MDM2 inhibitor that is under investigation in preclinical models for a variety of pediatric malignancies, including retinoblastoma, rhabdomyosarcoma, neuroblastoma, and leukemia. We used physiologically based pharmacokinetic (PBPK) modeling to characterize the disposition of nutlin-3a in the mouse. Plasma protein binding and blood partitioning were assessed by in vitro studies. After intravenous (10 and 20 mg/kg) and oral (50, 100, and 200 mg/kg) dosing, tissue concentrations of nutlin-3a were determined in plasma, liver, spleen, intestine, muscle, lung, adipose, bone marrow, adrenal gland, brain, retina, and vitreous fluid. The PBPK model was simultaneously fit to all pharmacokinetic data using NONMEM. Nutlin-3a exhibited nonlinear binding to murine plasma proteins, with the unbound fraction ranging from 0.7 to 11.8%. Nutlin-3a disposition was characterized by rapid absorption with peak plasma concentrations at approximately 2 h and biphasic elimination consistent with a saturable clearance process. The final PBPK model successfully described the plasma and tissue disposition of nutlin-3a. Simulations suggested high bioavailability, rapid attainment of steady state, and little accumulation when administered once or twice daily at dosages up to 400 mg/kg. The final model was used to perform simulations of unbound tissue concentrations to determine which dosing regimens are appropriate for preclinical models of several pediatric malignancies.
Figures
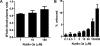



References
-
- Amemiya T, Yoshida H, Ishigooka H. (1979) Vitreous seeds in retinoblastoma, clinical significance and ultrastructure. Albrecht Von Graefes Arch Klin Exp Ophthalmol 211:205–213 - PubMed
-
- Barbieri A, Sabatini L, Indiveri P, Bonfiglioli R, Lodi V, Violante FS. (2006a) Simultaneous determination of low levels of methotrexate and cyclophosphamide in human urine by micro liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 20:1889–1893 - PubMed
-
- Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S, Shohet JM. (2006b) MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther 5:2358–2365 - PubMed
-
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

