Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex
- PMID: 20947702
- PMCID: PMC3025513
- DOI: 10.1152/ajpgi.00363.2010
Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex
Abstract
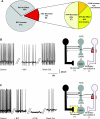
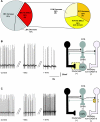
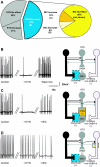
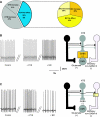
The dorsal motor nucleus of the vagus (DMV) is pivotal in the regulation of upper gastrointestinal functions, including motility and both gastric and pancreatic secretion. DMV neurons receive robust GABA- and glutamatergic inputs. Microinjection of the GABA(A) antagonist bicuculline (BIC) into the DMV increases pancreatic secretion and gastric motility, whereas the glutamatergic antagonist kynurenic acid (KYN) is ineffective unless preceded by microinjection of BIC. We used whole cell patch-clamp recordings with the aim of unveiling the brain stem neurocircuitry that uses tonic GABA- and glutamatergic synapses to control the activity of DMV neurons in a brain stem slice preparation. Perfusion with BIC altered the firing frequency of 71% of DMV neurons, increasing firing frequency in 80% of the responsive neurons and decreasing firing frequency in 20%. Addition of KYN to the perfusate either decreased (52%) or increased (25%) the firing frequency of BIC-sensitive neurons. When KYN was applied first, the firing rate was decreased in 43% and increased in 21% of the neurons; further perfusion with BIC had no additional effect in the majority of neurons. Our results indicate that there are several permutations in the arrangements of GABA- and glutamatergic inputs controlling the activity of DMV neurons. Our data support the concept of brain stem neuronal circuitry that may be wired in a finely tuned organ- or function-specific manner that permits precise and discrete modulation of the vagal motor output to the gastrointestinal tract.
Figures



References
-
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989 - PubMed
-
- Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 259: H1307–H1311, 1990 - PubMed
-
- Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol 77: 2539–2548, 1997 - PubMed
-
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol 59: 814–824, 2001 - PubMed
-
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

