Combination 5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301)
- PMID: 20947887
- PMCID: PMC2981019
- DOI: 10.1136/gut.2010.216135
Combination 5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301)
Abstract
Purpose: Gemcitabine is the standard chemotherapy for patients with metastatic pancreatic adenocarcinoma. Although the 5-fluorouracil (5FU), folinic acid and cisplatin combination (LV5FU2-CDDP) is an option, the optimal order of the regimens must be determined. The first strategic phase III trial comparing LV5FU2-CDDP followed by gemcitabine versus gemcitabine followed by LV5FU2-CDDP was conducted.
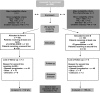
Methods: Patients with metastatic pancreatic adenocarcinoma, performance status (PS) 0-2, without prior chemotherapy were randomly assigned (1:1) to receive either LV5FU2-CDDP followed by gemcitabine at disease progression or toxicity (Arm A), or the opposite sequence (Arm B). 202 patients had to be included and 170 deaths had to be observed to detect an expected improvement in median overall survival (OS) from 6.5 to 10 months in Arm A (two-sided α = 5% and β = 20%).
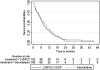
Results: 202 patients were included (Arm A, 102; Arm B, 100). Median age, male/female ratio, PS 0-1 and previous surgery were similar in the two arms. After a median follow-up of 44 months, median OS in Arm A was 6.6 months versus 8.0 months in Arm B (p = 0.85). Median progression-free survival was similar between Arms A and B. More grade 3/4 toxicities were observed when LV5FU2-CDDP was administered as a first-line treatment compared with gemcitabine: 79% versus 64% (p = 0.018).
Conclusion: This trial did not show any strategic advantage to using LV5FU2-CDDP as a first-line treatment and suggests that gemcitabine remains the standard first-line treatment. Sixty-one per cent of patients were able to receive a second line of chemotherapy.
Conflict of interest statement
Figures

Comment in
-
Optimal treatment of metastatic pancreatic cancer.Gut. 2010 Nov;59(11):1454-5. doi: 10.1136/gut.2010.220954. Gut. 2010. PMID: 20947879 No abstract available.
-
Pancreatic cancer: Gemcitabine confirmed as the first-line therapy for pancreatic cancer.Nat Rev Gastroenterol Hepatol. 2011 Jan;8(1):3. doi: 10.1038/nrgastro.2010.200. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21265057 No abstract available.
References
-
- Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer 1987;60:2284–303 - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13 - PubMed
-
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509–16 - PubMed
-
- Abou Alfa GK, Letourneau R, Harker WG, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol 2006;24:4441–7 - PubMed
-
- Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004;22:3776–83 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
