Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication
- PMID: 20949077
- PMCID: PMC2951379
- DOI: 10.1371/journal.ppat.1001141
Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication
Abstract
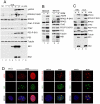
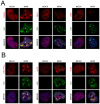
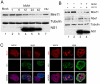
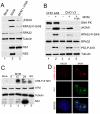
Infection by DNA viruses can elicit DNA damage responses (DDRs) in host cells. In some cases the DDR presents a block to viral replication that must be overcome, and in other cases the infecting agent exploits the DDR to facilitate replication. We find that low multiplicity infection with the autonomous parvovirus minute virus of mice (MVM) results in the activation of a DDR, characterized by the phosphorylation of H2AX, Nbs1, RPA32, Chk2 and p53. These proteins are recruited to MVM replication centers, where they co-localize with the main viral replication protein, NS1. The response is seen in both human and murine cell lines following infection with either the MVMp or MVMi strains. Replication of the virus is required for DNA damage signaling. Damage response proteins, including the ATM kinase, accumulate in viral-induced replication centers. Using mutant cell lines and specific kinase inhibitors, we show that ATM is the main transducer of the signaling events in the normal murine host. ATM inhibitors restrict MVM replication and ameliorate virus-induced cell cycle arrest, suggesting that DNA damage signaling facilitates virus replication, perhaps in part by promoting cell cycle arrest. Thus it appears that MVM exploits the cellular DNA damage response machinery early in infection to enhance its replication in host cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Lilley CE, Schwartz RA, Weitzman MD. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 2007;15:119–126. - PubMed
-
- Weitzman MD, Carson CT, Schwartz RA, Lilley CE. Interactions of viruses with the cellular DNA repair machinery. DNA Repair (Amst) 2004;3:1165–1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

