Recombinant adeno-associated virus type 2 pseudotypes: comparing safety, specificity, and transduction efficiency in the primate striatum. Laboratory investigation
- PMID: 20950087
- PMCID: PMC3773229
- DOI: 10.3171/2010.8.JNS091583
Recombinant adeno-associated virus type 2 pseudotypes: comparing safety, specificity, and transduction efficiency in the primate striatum. Laboratory investigation
Abstract
Object: Although several clinical trials utilizing the adeno-associated virus (AAV) type 2 serotype 2 (2/2) are now underway, it is unclear whether this particular serotype offers any advantage over others in terms of safety or efficiency when delivered directly to the CNS.
Methods: Recombinant AAV2-green fluorescent protein (GFP) serotypes 2/1, 2/2, 2/5, and 2/8 were generated following standard triple transfection protocols (final yield 5.4 × 10(12) particles/ml). A total of 180 μl of each solution was stereotactically infused, covering the entire rostrocaudal extent of the caudoputamen in 4 rhesus monkeys (Macaca mulatta) (3.0 ± 0.5 kg). After 6 weeks' survival, the brain was formalin fixed, cut at 40 μm, and stained with standard immunohistochemistry for anti-GFP, anticaspase-2, and cell-specific markers (anti-microtubule-associated protein-2 for neurons and anti-glial fibrillary acidic protein for glia). Unbiased stereological counting methods were used to determine cell number and striatal volume.
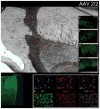
Results: The entire striatum of each animal contained GFP-positive cells with significant labeling extending beyond the borders of the basal ganglia. No ischemic/necrotic, hemorrhagic, or neoplastic change was observed in any brain. Total infusate volumes were similar across the 4 serotypes. However, GFP-labeled cell density was markedly different. Adeno-associated virus 2/1, 2/2, and 2/5 each labeled < 8000 cells/mm(3), whereas serotype 8 labeled > 21,000 cells, a 3- to 4-fold higher transduction efficiency. On the other hand, serotype 8 also labeled neurons and glia with equal affinity compared with neuronal specificities > 89% for the other serotypes. Moderate caspase-2 colabeling was noted in neurons immediately around the AAV2/1 injection tracts, but was not seen above the background anywhere in the brain following injections with serotypes 2, 5, or 8.
Conclusions: Intrastriatal delivery of AAV2 yields the highest cell transduction efficiencies but lowest neuronal specificity for serotype 8 when compared with serotypes 1, 2, and 5. Only AAV2/1 revealed significant caspase-2 activation. Careful consideration of serotype-specific differences in AAV2 neurotropism, transduction efficiency, and potential toxicity may affect future human trials.
Figures
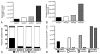


Comment in
-
Why should we care?J Neurosurg. 2011 Mar;114(3):670; discussion 670-1. doi: 10.3171/2010.7.JNS10767. Epub 2010 Oct 15. J Neurosurg. 2011. PMID: 20950078 No abstract available.
References
-
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. - PubMed
-
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. - PubMed
-
- Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. - PubMed
-
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudo-typed with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

