Teriparatide and osseous regeneration in the oral cavity
- PMID: 20950166
- PMCID: PMC5695223
- DOI: 10.1056/NEJMoa1005361
Teriparatide and osseous regeneration in the oral cavity
Abstract
Background: Intermittent administration of teriparatide, a drug composed of the first 34 amino acids of parathyroid hormone, has anabolic effects on bone. Although teriparatide has been evaluated for the treatment of osteoporosis and for the healing of fractures, clinical trials evaluating it for the treatment of osseous conditions of the oral cavity in humans are lacking.
Methods: A total of 40 patients with severe, chronic periodontitis underwent periodontal surgery and received daily injections of teriparatide (20 μg) or placebo, along with oral calcium (1000 mg) and vitamin D (800 IU) supplementation, for 6 weeks. The patients were followed for 1 year. The primary outcome was a radiographic linear measurement of alveolar bone level. Secondary outcomes included clinical variables, bone turnover markers in serum and oral fluid, systemic bone mineral density, and quality of life.
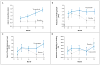
Results: Radiographic linear resolution of osseous defects was significantly greater after teriparatide therapy than after placebo beginning at 6 months, with a mean linear gain in bone at 1 year of 29% as compared with 3% (P<0.001). Clinical improvement was greater in patients taking teriparatide than in those taking placebo, with a reduction in periodontal probing depth of 33% versus 20% (2.42 mm vs. 1.32 mm) and a gain in clinical attachment level of 22% versus 7% (1.58 mm vs. 0.42 mm) in target lesions at 1 year (P = 0.02 for both comparisons). No serious adverse events were reported; however, the number of patients in the study was small. No significant differences were noted with respect to the other variables that were assessed.
Conclusions: Teriparatide, as compared with placebo, was associated with improved clinical outcomes, greater resolution of alveolar bone defects, and accelerated osseous wound healing in the oral cavity. Teriparatide may offer therapeutic potential for localized bone defects in the jaw. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT00277706 .).
Figures

Comment in
-
Teriparatide for bone loss in the jaw.N Engl J Med. 2010 Dec 16;363(25):2458-9. doi: 10.1056/NEJMe1010459. Epub 2010 Oct 16. N Engl J Med. 2010. PMID: 20950165 No abstract available.
-
Bone: Teriparatide improves outcomes of periodontal surgery.Nat Rev Endocrinol. 2011 Jan;7(1):4. doi: 10.1038/nrendo.2010.208. Nat Rev Endocrinol. 2011. PMID: 21197707 No abstract available.
-
Teriparatide and osseous regeneration in the jaw.N Engl J Med. 2011 Mar 17;364(11):1080; author reply 1080-1. doi: 10.1056/NEJMc1100728. N Engl J Med. 2011. PMID: 21410380 No abstract available.
References
-
- Curtis JW., Jr Infections associated with diabetes mellitus. N Engl J Med. 2000;342:895–6. - PubMed
-
- Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–94. - PubMed
-
- Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–20. - PubMed
-
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. - PubMed
-
- Offenbacher S, Beck JD, Moss K, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multi-centered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80:190–201. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
