shRNA knockdown of guanylate cyclase 2e or cyclic nucleotide gated channel alpha 1 increases photoreceptor survival in a cGMP phosphodiesterase mouse model of retinitis pigmentosa
- PMID: 20950332
- PMCID: PMC3071858
- DOI: 10.1111/j.1582-4934.2010.01201.x
shRNA knockdown of guanylate cyclase 2e or cyclic nucleotide gated channel alpha 1 increases photoreceptor survival in a cGMP phosphodiesterase mouse model of retinitis pigmentosa
Abstract
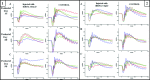
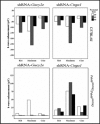
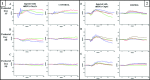
In vertebrate rods, dark and light conditions produce changes in guanosine 3',5'-cyclic monophosphate (cGMP) and calcium (Ca(2+) ) levels, which are regulated by the opposing function of several proteins. During the recovery of a bright flash, guanylate cyclase (GUCY) helps raise cGMP to levels that open cGMP-gated calcium sodium channels (CNG) to increase Na(+) and Ca(2+) influx in the outer segment. In contrast, light activates cGMP phosphodiesterase 6 (PDE6) causing rapid hydrolysis of cGMP, CNG closure, and reduced Na(+) and Ca(2+) levels. In Pde6b mouse models of retinitis pigmentosa (RP), photoreceptor death is preceded by abnormally high cGMP and Ca(2+) levels, likely because of continued synthesis of cGMP by guanylate cyclases and unregulated influx of Ca(2+) to toxic levels through CNG channels. To reverse the effects of Pde6b loss of function, we employed an shRNA knockdown approach to reduce the expression of Gucy2e or Cnga1 in Pde6b(H620Q) photoreceptors prior to degeneration. Gucy2e- or Cnga1-shRNA lentiviral-mediated knockdown GUCY2E and CNGA1 expression increase visual function and photoreceptor survival in Pde6b(H620Q) mice. We demonstrated that effective knockdown of GUCY2E and CNGA1 expression to counteract loss of PDE6 function may develop into a valuable approach for treating some patients with RP.
© 2011 The Authors Journal compilation © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Dryja TP, Berson EL. Retinitis pigmentosa and allied diseases: implications of genetic heterogeneity. Invest Opthal Vis Sci. 1995;36:1197–200. - PubMed
-
- Berson EL. Retinitis Pigmentosa: the Friedenwald Lecture. Invest Opthal Vis Sci. 1993;34:1655–76. - PubMed
-
- Bird AC. Retinal photoreceptor dystrophies: the LI. Edward Jackson Memorial Lecture. Am J Opthalmol. 1995;119:543–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

