Large volume unresectable locally advanced non-small cell lung cancer: acute toxicity and initial outcome results with rapid arc
- PMID: 20950469
- PMCID: PMC2972299
- DOI: 10.1186/1748-717X-5-94
Large volume unresectable locally advanced non-small cell lung cancer: acute toxicity and initial outcome results with rapid arc
Abstract
Background: To report acute toxicity, initial outcome results and planning therapeutic parameters in radiation treatment of advanced lung cancer (stage III) with volumetric modulated arcs using RapidArc (RA).
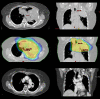
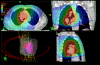
Methods: Twenty-four consecutive patients were treated with RA. All showed locally advanced non-small cell lung cancer with stage IIIA-IIIB and with large volumes (GTV:299 ± 175 cm3, PTV:818 ± 206 cm3). Dose prescription was 66Gy in 33 fractions to mean PTV. Delivery was performed with two partial arcs with a 6 MV photon beam.
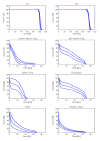
Results: From a dosimetric point of view, RA allowed us to respect most planning objectives on target volumes and organs at risk. In particular: for GTV D1% = 105.6 ± 1.7%, D99% = 96.7 ± 1.8%, D5%-D95% = 6.3 ± 1.4%; contra-lateral lung mean dose resulted in 13.7 ± 3.9Gy, for spinal cord D1% = 39.5 ± 4.0Gy, for heart V45Gy = 9.0 ± 7.0Gy, for esophagus D1% = 67.4 ± 2.2Gy. Delivery time was 133 ± 7s. At three months partial remission > 50% was observed in 56% of patients. Acute toxicities at 3 months showed 91% with grade 1 and 9% with grade 2 esophageal toxicity; 18% presented grade 1 and 9% with grade 2 pneumonia; no grade 3 acute toxicity was observed. The short follow-up does not allow assessment of local control and progression free survival.
Conclusions: RA proved to be a safe and advantageous treatment modality for NSCLC with large volumes. Long term observation of patients is needed to assess outcome and late toxicity.
Figures
References
-
- Jamal A, Twari RC, Murray T. et al.Cancer statistic. CAJ Clinic. 2004;54(8):29. - PubMed
-
- Graham MV, Pajak T, Herskovic A. et al.Preliminary results of a prospective trial using three dimensional radiotherapy for lung cancer. IJROPB. 1995;31:819–825.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

