Precise pattern of recombination in serotonergic and hypothalamic neurons in a Pdx1-cre transgenic mouse line
- PMID: 20950489
- PMCID: PMC2966455
- DOI: 10.1186/1423-0127-17-82
Precise pattern of recombination in serotonergic and hypothalamic neurons in a Pdx1-cre transgenic mouse line
Abstract
Background: Multicellular organisms are characterized by a remarkable diversity of morphologically distinct and functionally specialized cell types. Transgenic techniques for the manipulation of gene expression in specific cellular populations are highly useful for elucidating the development and function of these cellular populations. Given notable similarities in developmental gene expression between pancreatic β-cells and serotonergic neurons, we examined the pattern of Cre-mediated recombination in the nervous system of a widely used mouse line, Pdx1-cre (formal designation, Tg(Ipf1-cre)89.1Dam), in which the expression of Cre recombinase is driven by regulatory elements upstream of the pdx1 (pancreatic-duodenal homeobox 1) gene.
Methods: Single (hemizygous) transgenic mice of the pdx1-creCre/0 genotype were bred to single (hemizygous) transgenic reporter mice (Z/EG and rosa26R lines). Recombination pattern was examined in offspring using whole-mount and sectioned histological preparations at e9.5, e10.5, e11.5, e16.5 and adult developmental stages.
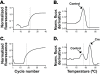
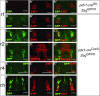
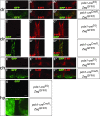
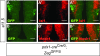
Results: In addition to the previously reported pancreatic recombination, recombination in the developing nervous system and inner ear formation was observed. In the central nervous system, we observed a highly specific pattern of recombination in neuronal progenitors in the ventral brainstem and diencephalon. In the rostral brainstem (r1-r2), recombination occurred in newborn serotonergic neurons. In the caudal brainstem, recombination occurred in non-serotonergic cells. In the adult, this resulted in reporter expression in the vast majority of forebrain-projecting serotonergic neurons (located in the dorsal and median raphe nuclei) but in none of the spinal cord-projecting serotonergic neurons of the caudal raphe nuclei. In the adult caudal brainstem, reporter expression was widespread in the inferior olive nucleus. In the adult hypothalamus, recombination was observed in the arcuate nucleus and dorsomedial hypothalamus. Recombination was not observed in any other region of the central nervous system. Neuronal expression of endogenous pdx1 was not observed.
Conclusions: The Pdx1-cre mouse line, and the regulatory elements contained in the corresponding transgene, could be a valuable tool for targeted genetic manipulation of developing forebrain-projecting serotonergic neurons and several other unique neuronal sub-populations. These results suggest that investigators employing this mouse line for studies of pancreatic function should consider the possible contributions of central nervous system effects towards resulting phenotypes.
Figures



Similar articles
-
Generation of Pet1210-Cre transgenic mouse line reveals non-serotonergic expression domains of Pet1 both in CNS and periphery.PLoS One. 2014 Aug 6;9(8):e104318. doi: 10.1371/journal.pone.0104318. eCollection 2014. PLoS One. 2014. PMID: 25098329 Free PMC article.
-
Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain.Diabetes. 2010 Dec;59(12):3090-8. doi: 10.2337/db10-0624. Epub 2010 Aug 29. Diabetes. 2010. PMID: 20802254 Free PMC article.
-
Cre-mediated recombination efficiency and transgene expression patterns of three retinal bipolar cell-expressing Cre transgenic mouse lines.Mol Vis. 2013 Jun 12;19:1310-20. Print 2013. Mol Vis. 2013. PMID: 23805038 Free PMC article.
-
The human raphe nuclei and the serotonergic system.J Chem Neuroanat. 2003 Dec;26(4):331-43. doi: 10.1016/j.jchemneu.2003.10.002. J Chem Neuroanat. 2003. PMID: 14729135 Review.
-
Mouse Cre Models for the Study of Bone Diseases.Curr Osteoporos Rep. 2018 Aug;16(4):466-477. doi: 10.1007/s11914-018-0455-7. Curr Osteoporos Rep. 2018. PMID: 29934753 Free PMC article. Review.
Cited by
-
Pancreas-specific Cre driver lines and considerations for their prudent use.Cell Metab. 2013 Jul 2;18(1):9-20. doi: 10.1016/j.cmet.2013.06.011. Cell Metab. 2013. PMID: 23823474 Free PMC article. Review.
-
Convergence of the insulin and serotonin programs in the pancreatic β-cell.Diabetes. 2011 Dec;60(12):3208-16. doi: 10.2337/db10-1192. Epub 2011 Oct 19. Diabetes. 2011. PMID: 22013016 Free PMC article.
-
Prohormone convertase 1/3 deficiency causes obesity due to impaired proinsulin processing.Nat Commun. 2022 Aug 13;13(1):4761. doi: 10.1038/s41467-022-32509-4. Nat Commun. 2022. PMID: 35963866 Free PMC article.
-
Pancreatic prolactin receptor signaling regulates maternal glucose homeostasis.J Endocrinol. 2019 Apr;241(1):71-83. doi: 10.1530/JOE-18-0518. J Endocrinol. 2019. PMID: 30798322 Free PMC article.
-
Hypothalamic Non-AgRP, Non-POMC GABAergic Neurons Are Required for Postweaning Feeding and NPY Hyperphagia.J Neurosci. 2015 Jul 22;35(29):10440-50. doi: 10.1523/JNEUROSCI.1110-15.2015. J Neurosci. 2015. PMID: 26203139 Free PMC article.
References
-
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72(1):165–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

