Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia
- PMID: 20950613
- PMCID: PMC3006487
- DOI: 10.1053/j.gastro.2010.09.048
Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia
Abstract
Background & aims: Transgenic FVB/N insulin-gastrin (INS-GAS) mice have high circulating gastrin levels, and develop spontaneous atrophic gastritis and gastrointestinal intraepithelial neoplasia (GIN) with 80% prevalence 6 months after Helicobacter pylori infection. GIN is associated with gastric atrophy and achlorhydria, predisposing mice to nonhelicobacter microbiota overgrowth. We determined if germfree INS-GAS mice spontaneously develop GIN and if H pylori accelerates GIN in gnotobiotic INS-GAS mice.
Methods: We compared gastric lesions, levels of messenger RNA, serum inflammatory mediators, antibodies, and gastrin among germfree and H pylori-monoinfected INS-GAS mice. Microbiota composition of specific pathogen-free (SPF) INS-GAS mice was quantified by pyrosequencing.
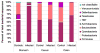
Results: Germfree INS-GAS mice had mild hypergastrinemia but did not develop significant gastric lesions until 9 months old and did not develop GIN through 13 months. H pylori monoassociation caused progressive gastritis, epithelial defects, oxyntic atrophy, marked foveolar hyperplasia, dysplasia, and robust serum and tissue proinflammatory immune responses (particularly males) between 5 and 11 months postinfection (P<0.05, compared with germfree controls). Only 2 of 26 female, whereas 8 of 18 male, H pylori-infected INS-GAS mice developed low to high-grade GIN by 11 months postinfection. Stomachs of H pylori-infected SPF male mice had significant reductions in Bacteroidetes and significant increases in Firmicutes.
Conclusions: Gastric lesions take 13 months longer to develop in germfree INS-GAS mice than male SPF INS-GAS mice. H pylori monoassociation accelerated gastritis and GIN but caused less severe gastric lesions and delayed onset of GIN compared with H pylori-infected INS-GAS mice with complex gastric microbiota. Changes in gastric microbiota composition might promote GIN in achlorhydric stomachs of SPF mice.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





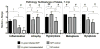
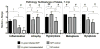
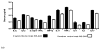
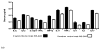
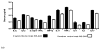
References
-
- Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. - PubMed
-
- Bruce MG, Maaroos HI. Epidemiology of Helicobacter pylori infection. Helicobacter. 2008;13 (Suppl 1):1–6. - PubMed
-
- Whary MT, Sundina N, Bravo LE, et al. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005;14:1464–1469. - PubMed
-
- Fox JG, Li X, Cahill RJ, et al. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

