Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries
- PMID: 20950853
- PMCID: PMC2995374
- DOI: 10.1016/j.biomaterials.2010.08.117
Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries
Abstract
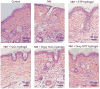
Doxycycline hydrogels containing reversible disulfide crosslinks were investigated for a dermal wound healing application. Nitrogen mustard (NM) was used as a surrogate to mimic the vesicant effects of the chemical warfare agent sulfur mustard. An 8-arm-poly(ethylene glycol) (PEG) polymer containing multiple thiol (-SH) groups was crosslinked using hydrogen peroxide (H(2)O(2) hydrogel) or 8-arm-S-thiopyridyl (S-TP hydrogel) to form a hydrogel in situ. Formulation additives (glycerin, PVP and PEG 600) were found to promote dermal hydrogel retention for up to 24 h. Hydrogels demonstrated high mechanical strength and a low degree of swelling (< 1.5%). Doxycycline release from the hydrogels was biphasic and sustained for up to 10-days in vitro. Doxycycline (8.5 mg/cm(3)) permeability through NM-exposed skin was elevated as compared to non vesicant-treated controls at 24, 72 and 168 h post-exposure with peak permeability at 72 h. The decrease in doxycycline permeability at 168 h correlates to epidermal re-epithelialization and wound healing. Histology studies of skin showed that doxycycline loaded (0.25% w/v) hydrogels provided improved wound healing response on NM-exposed skin as compared to untreated skin and skin treated with placebo hydrogels in an SKH-1 mouse model. In conclusion, PEG-based doxycycline hydrogels are promising for dermal wound healing application of mustard injuries.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures







References
-
- Shohrati M, Peyman M, Peyman A, Davoudi M, Ghanei M. Cutaneous and ocular late complications of sulfur mustard in Iranian veterans. Cutan Ocul Toxicol. 2007;26(2):73–81. - PubMed
-
- Isidore MA, Castagna MP, Steele KE, Gordon RK, Nambiar MP. A dorsal model for cutaneous vesicant injury by 2-chloroethyl ethyl sulfide using C57BL/6 mice. Cutan Ocul Toxicol. 2007;26(3):265–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

