Sclerostin-erbB-3 interactions: modulation of erbB-3 activity by sclerostin
- PMID: 20951118
- PMCID: PMC2992958
- DOI: 10.1016/j.bbrc.2010.10.048
Sclerostin-erbB-3 interactions: modulation of erbB-3 activity by sclerostin
Abstract
To gain insights into the mechanism of action of sclerostin, a protein that regulates bone mass, we performed yeast two-hybrid analyses using human SOST (sclerostin) cDNA cloned into pGBKT7 DNA-binding domain vector as a bait, and a normalized, high-complexity, universal cDNA library in a GAL4 activating domain vector. We identified an interaction between sclerostin and the carboxyl-terminal portion of the receptor tyrosine-protein kinase erbB-3. To determine the biological relevance of this interaction, we treated MC3T3-E1 mouse osteoblast cells transfected with either a SOST expression plasmid or a control vector, with recombinant heregulin/neuregulin. Phospho-p44/42 (Thr202/Tyr204) MAPK was assessed in heregulin/neuregulin treated cells. We observed an increase in phospho-p44/42 (Thr202/Tyr204) MAPK concentrations in SOST transfected cells but not in cells transfected with a control vector, thus demonstrating a modulatory effect of sclerostin on heregulin/neuregulin signaling in osteoblasts. The data demonstrate that sclerostin functions in part, by modulating the activity of erbB-3.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
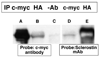

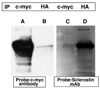

References
-
- Beighton PH, Hanmersma H, Brunkow ME. SOST-Related Sclerosing Bone Dysplasias. GeneReviews NCBI Bookshelf; 2010.
-
- Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A, Gardner JC, Galas D, Schatzman RC, Beighton P, Papapoulos S, Hamersma H, Brunkow ME. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. - PubMed
-
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

