Colitis and intestinal inflammation in IL10-/- mice results from IL-13Rα2-mediated attenuation of IL-13 activity
- PMID: 20951137
- PMCID: PMC3006653
- DOI: 10.1053/j.gastro.2010.09.047
Colitis and intestinal inflammation in IL10-/- mice results from IL-13Rα2-mediated attenuation of IL-13 activity
Abstract
Background & aims: The cytokine interleukin (IL)-10 is required to maintain immune homeostasis in the gastrointestinal tract. IL-10 null mice spontaneously develop colitis or are more susceptible to induction of colitis by infections, drugs, and autoimmune reactions. IL-13 regulates inflammatory conditions; its activity might be compromised by the IL-13 decoy receptor (IL-13Rα2).
Methods: We examined the roles of IL-13 and IL-13Rα2 in intestinal inflammation in mice. To study the function of IL-13Rα2, il10(-/-) mice were crossed with il13rα2(-/-) to generate il10(-/-)il13rα2(-/-) double knockout (dKO) mice. Colitis was induced with the gastrointestinal toxin piroxicam or Trichuris muris infection.
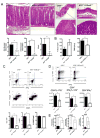
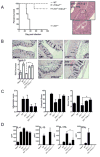
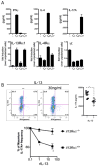
Results: Induction of colitis by interferon (IFN)-γ or IL-17 in IL-10 null mice requires IL-13Rα2. Following exposure of il10(-/-) mice to piroxicam or infection with T muris, production of IL-13Rα2 increased, resulting in decreased IL-13 bioactivity and increased inflammation in response to IFN-γ or IL-17A. In contrast to il10(-/-) mice, dKO mice were resistant to piroxicam-induced colitis; they also developed less severe colitis during chronic infection with T muris infection. In both models, resistance to IFN-γ and IL-17-mediated intestinal inflammation was associated with increased IL-13 activity. Susceptibility to colitis was restored when the dKO mice were injected with monoclonal antibodies against IL-13, confirming its protective role.
Conclusions: Colitis and intestinal inflammation in IL10(-/-) mice results from IL-13Rα2-mediated attenuation of IL-13 activity. In the absence of IL-13Rα2, IL-13 suppresses proinflammatory Th1 and Th17 responses. Reagents that block the IL-13 decoy receptor IL-13Rα2 might be developed for inflammatory bowel disease associated with increased levels of IFN-γ and IL-17.
Published by Elsevier Inc.
Figures

Similar articles
-
KSR1 protects from interleukin-10 deficiency-induced colitis in mice by suppressing T-lymphocyte interferon-γ production.Gastroenterology. 2011 Jan;140(1):265-74. doi: 10.1053/j.gastro.2010.09.041. Epub 2010 Sep 25. Gastroenterology. 2011. PMID: 20875416 Free PMC article.
-
Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation.Infect Immun. 2001 Jul;69(7):4232-41. doi: 10.1128/IAI.69.7.4232-4241.2001. Infect Immun. 2001. PMID: 11401959 Free PMC article.
-
The innate antiviral response upregulates IL-13 receptor α2 in bronchial fibroblasts.J Allergy Clin Immunol. 2013 Mar;131(3):849-55. doi: 10.1016/j.jaci.2012.08.030. Epub 2012 Oct 12. J Allergy Clin Immunol. 2013. PMID: 23069489
-
Ablation of IL-17A leads to severe colitis in IL-10-deficient mice: implications of myeloid-derived suppressor cells and NO production.Int Immunol. 2020 Mar 7;32(3):187-201. doi: 10.1093/intimm/dxz076. Int Immunol. 2020. PMID: 31755523 Free PMC article.
-
The signaling function of the IL-13Ralpha2 receptor in the development of gastrointestinal fibrosis and cancer surveillance.Curr Mol Med. 2009 Aug;9(6):740-50. doi: 10.2174/156652409788970652. Curr Mol Med. 2009. PMID: 19689301 Free PMC article. Review.
Cited by
-
Translatability of helminth therapy in inflammatory bowel diseases.Int J Parasitol. 2013 Mar;43(3-4):245-51. doi: 10.1016/j.ijpara.2012.10.016. Epub 2012 Nov 21. Int J Parasitol. 2013. PMID: 23178819 Free PMC article. Review.
-
Lymph Node Stromal Cells From Different Draining Areas Distinctly Regulate the Development of Chronic Intestinal Inflammation.Front Immunol. 2021 Feb 16;11:549473. doi: 10.3389/fimmu.2020.549473. eCollection 2020. Front Immunol. 2021. PMID: 33664727 Free PMC article.
-
IL-13 receptor α2-arginase 2 pathway mediates IL-13-induced pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2013 Jan 15;304(2):L112-24. doi: 10.1152/ajplung.00101.2012. Epub 2012 Nov 2. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23125252 Free PMC article.
-
Type 2 cytokines: mechanisms and therapeutic strategies.Nat Rev Immunol. 2015 May;15(5):271-82. doi: 10.1038/nri3831. Epub 2015 Apr 17. Nat Rev Immunol. 2015. PMID: 25882242 Review.
-
Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance.Nat Commun. 2011 Feb 8;2:190. doi: 10.1038/ncomms1181. Nat Commun. 2011. PMID: 21304519 Free PMC article.
References
-
- Kelsall BL. Innate and adaptive mechanisms to control [corrected] pathological intestinal inflammation. J Pathol. 2008;214:242–59. - PubMed
-
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–38. - PubMed
-
- Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1:252–64. - PubMed
-
- Cucino C, Sonnenberg A. Cause of death in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:250–5. - PubMed
-
- Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

