Cholesterol is important for a post-adsorption step in the entry process of transmissible gastroenteritis virus
- PMID: 20951168
- PMCID: PMC7114218
- DOI: 10.1016/j.antiviral.2010.10.002
Cholesterol is important for a post-adsorption step in the entry process of transmissible gastroenteritis virus
Abstract
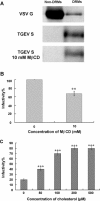
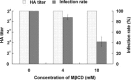
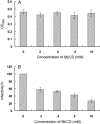
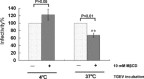
Cholesterol is a major constituent of detergent-resistant membrane microdomains (DRMs). We localized transmissible gastroenteritis virus (TGEV) spike (S) protein in DRMs in the viral envelope. Though S protein was not solubilized by cold non-ionic detergents, this behavior was unchanged when cholesterol was depleted from viral membrane by methyl-β-cyclodextrin (MβCD) and the protein did not comigrate with cellular DRM marker proteins in flotation analyses. Therefore, the S protein is not anchored in the viral membrane DRMs as they are known to occur in the plasma membrane. Cholesterol depletion from viral membrane may not affect the adsorption process as neither the sialic acid binding activity nor the binding to aminopeptidase N was reduced post-MβCD treatment. Reduced infectivity of cholesterol-depleted TGEV was observed only when the adsorption process occurred at 37°C but not when the virus was applied at 4°C. Cholesterol is important for a post-adsorption step, allowing membrane rearrangements that facilitate virus entry.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Importance of cholesterol for infection of cells by transmissible gastroenteritis virus.Virus Res. 2008 Nov;137(2):220-4. doi: 10.1016/j.virusres.2008.07.023. Epub 2008 Sep 16. Virus Res. 2008. PMID: 18727942 Free PMC article.
-
The sialic acid binding activity of the S protein facilitates infection by porcine transmissible gastroenteritis coronavirus.Virol J. 2011 Sep 12;8:435. doi: 10.1186/1743-422X-8-435. Virol J. 2011. PMID: 21910859 Free PMC article.
-
Dynamics of transmissible gastroenteritis virus internalization unraveled by single-virus tracking in live cells.FASEB J. 2020 Mar;34(3):4653-4669. doi: 10.1096/fj.201902455R. Epub 2020 Feb 4. FASEB J. 2020. PMID: 32017270 Free PMC article.
-
Binding of transmissible gastroenteritis coronavirus to brush border membrane sialoglycoproteins.J Virol. 2003 Nov;77(21):11846-8. doi: 10.1128/jvi.77.21.11846-11848.2003. J Virol. 2003. PMID: 14557669 Free PMC article.
-
Transmissible gastroenteritis virus infection: a vanishing specter.Dtsch Tierarztl Wochenschr. 2006 Apr;113(4):157-9. Dtsch Tierarztl Wochenschr. 2006. PMID: 16716052 Review.
Cited by
-
Phage displayed peptides recognizing porcine aminopeptidase N inhibit transmissible gastroenteritis coronavirus infection in vitro.Virology. 2011 Feb 20;410(2):299-306. doi: 10.1016/j.virol.2010.11.014. Epub 2010 Dec 21. Virology. 2011. PMID: 21176936 Free PMC article.
-
Transmissible gastroenteritis virus: identification of M protein-binding peptide ligands with antiviral and diagnostic potential.Antiviral Res. 2013 Sep;99(3):383-90. doi: 10.1016/j.antiviral.2013.06.015. Epub 2013 Jul 2. Antiviral Res. 2013. PMID: 23830854 Free PMC article.
-
Research Advances on the Role of Lipids in the Life Cycle of Human Coronaviruses.Microorganisms. 2023 Dec 28;12(1):63. doi: 10.3390/microorganisms12010063. Microorganisms. 2023. PMID: 38257890 Free PMC article. Review.
-
Transferrin receptor 1 levels at the cell surface influence the susceptibility of newborn piglets to PEDV infection.PLoS Pathog. 2020 Jul 30;16(7):e1008682. doi: 10.1371/journal.ppat.1008682. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32730327 Free PMC article.
-
A Review of Bioactive Compounds against Porcine Enteric Coronaviruses.Viruses. 2022 Oct 8;14(10):2217. doi: 10.3390/v14102217. Viruses. 2022. PMID: 36298772 Free PMC article. Review.
References
-
- Delmas B., Rasschaert D., Godet M., Gelfi J., Laude H. Four major antigenic sites of the coronavirus transmissible gastroenteritis virus are located on the amino-terminal half of spike glycoprotein S. J. Gen. Virol. 1990;71:1313–1323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

