Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration
- PMID: 20951206
- PMCID: PMC3014407
- DOI: 10.1016/j.nbd.2010.10.009
Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration
Abstract
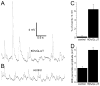
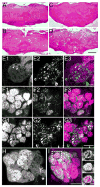
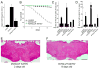
Increases in vesicular glutamate transporter (VGLUT) levels are observed after a variety of insults including hypoxic injury, stress, methamphetamine treatment, and in genetic seizure models. Such overexpression can cause an increase in the amount of glutamate released from each vesicle, but it is unknown whether this is sufficient to induce excitotoxic neurodegeneration. Here we show that overexpression of the Drosophila vesicular glutamate transporter (DVGLUT) leads to excess glutamate release, with some vesicles releasing several times the normal amount of glutamate. Increased DVGLUT expression also leads to an age-dependent loss of motor function and shortened lifespan, accompanied by a progressive neurodegeneration in the postsynaptic targets of the DVGLUT-overexpressing neurons. The early onset lethality, behavioral deficits, and neuronal pathology require overexpression of a functional DVGLUT transgene. Thus overexpression of DVGLUT is sufficient to generate excitotoxic neuropathological phenotypes and therefore reducing VGLUT levels after nervous system injury or stress may mitigate further damage.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
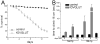
Similar articles
-
Na+ /H+ exchange via the Drosophila vesicular glutamate transporter mediates activity-induced acid efflux from presynaptic terminals.J Physiol. 2017 Feb 1;595(3):805-824. doi: 10.1113/JP273105. Epub 2016 Nov 13. J Physiol. 2017. PMID: 27641622 Free PMC article.
-
A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle.Neuron. 2006 Jan 5;49(1):11-6. doi: 10.1016/j.neuron.2005.11.032. Neuron. 2006. PMID: 16387635 Free PMC article.
-
The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain.Gene Expr Patterns. 2006 Mar;6(3):299-309. doi: 10.1016/j.modgep.2005.07.006. Epub 2005 Dec 27. Gene Expr Patterns. 2006. PMID: 16378756
-
VGLUTs: 'exciting' times for glutamatergic research?Neurosci Res. 2006 Aug;55(4):343-51. doi: 10.1016/j.neures.2006.04.016. Epub 2006 Jun 9. Neurosci Res. 2006. PMID: 16765470 Review.
-
Synaptic vesicle protein trafficking at the glutamate synapse.Neuroscience. 2009 Jan 12;158(1):189-203. doi: 10.1016/j.neuroscience.2008.03.029. Epub 2008 Mar 22. Neuroscience. 2009. PMID: 18472224 Free PMC article. Review.
Cited by
-
SLC transporters as therapeutic targets: emerging opportunities.Nat Rev Drug Discov. 2015 Aug;14(8):543-60. doi: 10.1038/nrd4626. Epub 2015 Jun 26. Nat Rev Drug Discov. 2015. PMID: 26111766 Free PMC article. Review.
-
Overnutrition during Pregnancy and Lactation Induces Gender-Dependent Dysmetabolism in the Offspring Accompanied by Heightened Stress and Anxiety.Nutrients. 2023 Dec 25;16(1):67. doi: 10.3390/nu16010067. Nutrients. 2023. PMID: 38201896 Free PMC article.
-
Vesicular neurotransmitter transporters in Drosophila melanogaster.Biochim Biophys Acta Biomembr. 2020 Dec 1;1862(12):183308. doi: 10.1016/j.bbamem.2020.183308. Epub 2020 Apr 17. Biochim Biophys Acta Biomembr. 2020. PMID: 32305263 Free PMC article. Review.
-
Na+ /H+ exchange via the Drosophila vesicular glutamate transporter mediates activity-induced acid efflux from presynaptic terminals.J Physiol. 2017 Feb 1;595(3):805-824. doi: 10.1113/JP273105. Epub 2016 Nov 13. J Physiol. 2017. PMID: 27641622 Free PMC article.
-
Effect size of memory deficits in mice with adult-onset P301L tau expression.Behav Brain Res. 2014 Oct 1;272:181-95. doi: 10.1016/j.bbr.2014.06.057. Epub 2014 Jul 6. Behav Brain Res. 2014. PMID: 25004446 Free PMC article.
References
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. - PubMed
-
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in the Drosophila larval and adult CNS. J Comp Neurol. 2008;508(1):131–152. - PubMed
-
- DiAntonio A. Glutamate receptors at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:165–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

