Kinetochores' gripping feat: conformational wave or biased diffusion?
- PMID: 20951587
- PMCID: PMC3075839
- DOI: 10.1016/j.tcb.2010.09.003
Kinetochores' gripping feat: conformational wave or biased diffusion?
Abstract
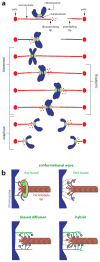
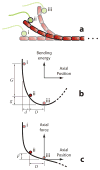
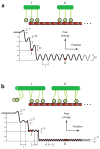
Climbing up a cliff while the rope unravels underneath your fingers does not sound like a well-planned adventure. Yet chromosomes face a similar challenge during each cell division. Their alignment and accurate segregation depends on staying attached to the assembling and disassembling tips of microtubule fibers. This coupling is mediated by kinetochores, intricate machines that attach chromosomes to an ever-changing microtubule substrate. Two models for kinetochore-microtubule coupling were proposed a quarter century ago: conformational wave and biased diffusion. These models differ in their predictions for how coupling is performed and regulated. The availability of purified kinetochore proteins has enabled biochemical and biophysical analyses of the kinetochore-microtubule interface. Here, we discuss what these studies reveal about the contributions of each model.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- McAinsh AD, et al. Structure, function, and regulation of budding yeast kinetochores. Annu Rev Cell Dev Biol. 2003;19:519–539. - PubMed
-
- Westermann S, et al. Structures and functions of yeast kinetochore complexes. Annu Rev Biochem. 2007;76:563–591. - PubMed
-
- Miranda JJ, et al. The yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol. 2005;12:138–143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

