T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation
- PMID: 20951945
- PMCID: PMC3003429
- DOI: 10.1016/j.ccr.2010.09.009
T-lymphoblastic lymphoma cells express high levels of BCL2, S1P1, and ICAM1, leading to a blockade of tumor cell intravasation
Erratum in
-
T-Lymphoblastic Lymphoma Cells Express High Levels of BCL2, S1P1, and ICAM1, Leading to a Blockade of Tumor Cell Intravasation.Cancer Cell. 2024 Jun 10;42(6):1130-1131. doi: 10.1016/j.ccell.2024.05.019. Cancer Cell. 2024. PMID: 38861925 Free PMC article. No abstract available.
Abstract
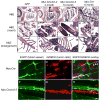
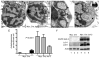
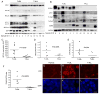
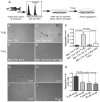
The molecular events underlying the progression of T-lymphoblastic lymphoma (T-LBL) to acute T-lymphoblastic leukemia (T-ALL) remain elusive. In our zebrafish model, concomitant overexpression of bcl-2 with Myc accelerated T-LBL onset while inhibiting progression to T-ALL. The T-LBL cells failed to invade the vasculature and showed evidence of increased homotypic cell-cell adhesion and autophagy. Further analysis using clinical biopsy specimens revealed autophagy and increased levels of BCL2, S1P1, and ICAM1 in human T-LBL compared with T-ALL. Inhibition of S1P1 signaling in T-LBL cells led to decreased homotypic adhesion in vitro and increased tumor cell intravasation in vivo. Thus, blockade of intravasation and hematologic dissemination in T-LBL is due to elevated S1P1 signaling, increased expression of ICAM1, and augmented homotypic cell-cell adhesion.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




Comment in
-
T-lineage lymphoblastic lymphoma and leukemia-a MASSive problem.Cancer Cell. 2010 Oct 19;18(4):297-9. doi: 10.1016/j.ccr.2010.10.003. Cancer Cell. 2010. PMID: 20951938
References
-
- Asker C, Wiman KG, Selivanova G. p53-induced apoptosis as a safeguard against cancer. Biochem Biophys Res Commun. 1999;265:1–6. - PubMed
-
- Bernard G, Zoccola D, Deckert M, Breittmayer JP, Aussel C, Bernard A. The E2 molecule (CD99) specifically triggers homotypic aggregation of CD4+ CD8+ thymocytes. J Immunol. 1995;154(1):26–32. - PubMed
-
- Cairo MS, Raetz E, Lim MS, Davenport V, Perkins SL. Childhood and adolescent non-Hodgkin lymphoma: new insights in biology and critical challenges for the future. Pediatric blood & cancer. 2005;45:753–769. - PubMed
-
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1K08CA133103/CA/NCI NIH HHS/United States
- K01 DK074555/DK/NIDDK NIH HHS/United States
- K01AR05562190-01A1/AR/NIAMS NIH HHS/United States
- 3K01AR055619-03S1/AR/NIAMS NIH HHS/United States
- T32 HL086344/HL/NHLBI NIH HHS/United States
- K08 CA133103/CA/NCI NIH HHS/United States
- K99CA134743/CA/NCI NIH HHS/United States
- CA068484/CA/NCI NIH HHS/United States
- 1K01DK074555/DK/NIDDK NIH HHS/United States
- K01 AR055619/AR/NIAMS NIH HHS/United States
- K01 DK087814/DK/NIDDK NIH HHS/United States
- R00 CA134743/CA/NCI NIH HHS/United States
- K99 CA134743/CA/NCI NIH HHS/United States
- R01 CA077429/CA/NCI NIH HHS/United States
- T32-HL086344/HL/NHLBI NIH HHS/United States
- L40 CA124083/CA/NCI NIH HHS/United States
- CA077429/CA/NCI NIH HHS/United States
- P01 CA068484/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

