C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output
- PMID: 20952387
- PMCID: PMC3000950
- DOI: 10.1074/jbc.M110.180695
C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output
Abstract
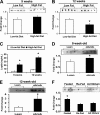
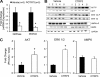
Adipose tissue-derived adipokines play important roles in controlling systemic insulin sensitivity and energy balance. Our recent efforts to identify novel metabolic mediators produced by adipose tissue have led to the discovery of a highly conserved family of secreted proteins, designated as C1q/TNF-related proteins 1-10 (CTRP1 to -10). However, physiological functions regulated by CTRPs are largely unknown. Here we provide the first in vivo functional characterization of CTRP3. We show that circulating levels of CTRP3 are inversely correlated with leptin levels; CTRP3 increases with fasting, decreases in diet-induced obese mice with high leptin levels, and increases in leptin-deficient ob/ob mice. A modest 3-fold elevation of plasma CTRP3 levels by recombinant protein administration is sufficient to lower glucose levels in normal and insulin-resistant ob/ob mice, without altering insulin or adiponectin levels. The glucose-lowering effect in mice is linked to activation of the Akt signaling pathway in liver and a marked suppression of hepatic gluconeogenic gene expression. Consistent with its effects in mice, CTRP3 acts directly and independently of insulin to regulate gluconeogenesis in cultured hepatocytes. In humans, alternative splicing generates two circulating CTRP3 isoforms differing in size and glycosylation pattern. The two human proteins form hetero-oligomers, an association that does not require interdisulfide bond formation and appears to protect the longer isoform from proteolytic cleavage. Recombinant human CTRP3 also reduces glucose output in hepatocytes by suppressing gluconeogenic enzyme expression. This study provides the first functional evidence linking CTRP3 to hepatic glucose metabolism and establishes CTRP3 as a novel adipokine.
Figures





References
-
- Scherer P. E. (2006) Diabetes 55, 1537–1545 - PubMed
-
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. (1994) Nature 372, 425–432 - PubMed
-
- Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. (1995) J. Biol. Chem. 270, 26746–26749 - PubMed
-
- Steppan C. M., Bailey S. T., Bhat S., Brown E. J., Banerjee R. R., Wright C. M., Patel H. R., Ahima R. S., Lazar M. A. (2001) Nature 409, 307–312 - PubMed
-
- Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Nature 436, 356–362 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

