The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain
- PMID: 20952483
- PMCID: PMC3014301
- DOI: 10.1124/jpet.110.175034
The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain
Abstract
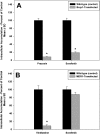
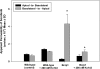
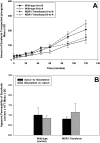
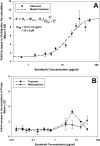
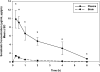
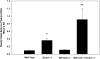
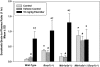
ATP-binding cassette transporters P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) have been shown to work in concert to restrict brain penetration of several tyrosine kinase inhibitors. It has been reported that P-gp is dominant in limiting transport of many dual P-gp/BCRP substrates across the blood-brain barrier (BBB). This study investigated the influence of P-gp and BCRP on the central nervous system (CNS) penetration of sorafenib, a multitargeted tyrosine kinase inhibitor currently being evaluated in clinical trials for glioma. In vitro studies showed that BCRP has a high affinity for sorafenib. Sorafenib inhibited P-gp, but did not seem to be a P-gp substrate in vitro. CNS distribution studies showed that transport of sorafenib to the brain was restricted because of active efflux at the BBB. The brain-to-plasma equilibrium-distribution coefficient (area under the concentration-time profiles for plasma/area under the concentration-time profiles for brain) was 0.06 in wild-type mice. Steady-state brain-to-plasma concentration ratio of sorafenib was approximately 0.36 ± 0.056 in the Bcrp1(-/-) mice, 0.11 ± 0.021 in the Mdr1a/b(-/-) mice, and 0.91 ± 0.29 in the Mdr1a/b(-/-)Bcrp1(-/-) mice compared with 0.094 ± 0.007 in the wild-type mice. Sorafenib brain-to-plasma ratios increased on coadministration of the dual P-gp/BCRP inhibitor elacridar such that the ratio in wild-type mice (0.76 ± 0.24), Bcrp1(-/-) mice (1.03 ± 0.33), Mdr1a/b(-/-) mice (1.3 ± 0.29), and Mdr1a/b(-/-)Bcrp1(-/-) mice (0.73 ± 0.35) were not significantly different. This study shows that BCRP and P-gp together restrict the brain distribution of sorafenib with BCRP playing a dominant role in the efflux of sorafenib at the BBB. These findings are clinically relevant to chemotherapy in glioma if restricted drug delivery to the invasive tumor cells results in decreased efficacy.
Figures


References
-
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1:417–425 - PubMed
-
- Bailer AJ. (1988) Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm 16:303–309 - PubMed
-
- Begley DJ. (2004) ABC transporters and the blood-brain barrier. Curr Pharm Des 10:1295–1312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

