p53 status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner
- PMID: 20952507
- PMCID: PMC2999653
- DOI: 10.1158/0008-5472.CAN-10-1146
p53 status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner
Abstract
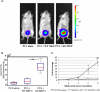
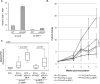
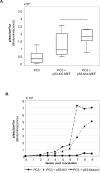
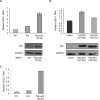
The p53 tumor suppressor exerts a variety of cell-autonomous effects that are aimed to thwart tumor development. In addition, however, there is growing evidence for cell nonautonomous tumor suppressor effects of p53. In the present study, we investigated the impact of stromal p53 on tumor growth. Specifically, we found that ablation of p53 in fibroblasts enabled them to promote more efficiently the growth of tumors initiated by PC3 prostate cancer-derived cells. This stimulatory effect was dependent on the increased expression of the chemokine SDF-1 in the p53-deficient fibroblasts. Notably, fibroblasts harboring mutant p53 protein were more effective than p53-null fibroblasts in promoting tumor growth. The presence of either p53-null or p53-mutant fibroblasts led also to a markedly elevated rate of metastatic spread of the PC3 tumors. These findings implicate p53 in a cell nonautonomous tumor suppressor role within stromal fibroblasts, through suppressing the production of tumor stimulatory factors by these cells. Moreover, expression of mutant p53 by tumor stroma fibroblasts might exert a gain of function effect, further accelerating tumor development.
Figures

References
-
- Hill R, et al. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123(6):1001–11. - PubMed
-
- Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11(1):54–9. - PubMed
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. - PubMed
-
- Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

