RAD51C: a novel cancer susceptibility gene is linked to Fanconi anemia and breast cancer
- PMID: 20952512
- PMCID: PMC2994284
- DOI: 10.1093/carcin/bgq210
RAD51C: a novel cancer susceptibility gene is linked to Fanconi anemia and breast cancer
Abstract
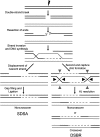
Germline mutations in many of the genes that are involved in homologous recombination (HR)-mediated DNA double-strand break repair (DSBR) are associated with various human genetic disorders and cancer. RAD51 and RAD51 paralogs are important for HR and in the maintenance of genome stability. Despite the identification of five RAD51 paralogs over a decade ago, the molecular mechanism(s) by which RAD51 paralogs regulate HR and genome maintenance remains obscure. In addition to the known roles of RAD51C in early and late stages of HR, it also contributes to activation of the checkpoint kinase CHK2. One recent study identifies biallelic mutation in RAD51C leading to Fanconi anemia-like disorder. Whereas a second study reports monoallelic mutation in RAD51C associated with increased risk of breast and ovarian cancer. These reports show RAD51C is a cancer susceptibility gene. In this review, we focus on describing the functions of RAD51C in HR, DNA damage signaling and as a tumor suppressor with an emphasis on the new roles of RAD51C unveiled by these reports.
Figures

References
-
- Branzei D, et al. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008;9:297–308. - PubMed
-
- Thompson LH, et al. Recombinational DNA repair and human disease. Mutat. Res. 2002;509:49–78. - PubMed
-
- Sung P, et al. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat. Rev. Mol. Cell Biol. 2006;7:739–750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

