Liposomal cytarabine is effective and tolerable in the treatment of central nervous system relapse of acute lymphoblastic leukemia and very aggressive lymphoma
- PMID: 20952517
- PMCID: PMC3031691
- DOI: 10.3324/haematol.2010.028092
Liposomal cytarabine is effective and tolerable in the treatment of central nervous system relapse of acute lymphoblastic leukemia and very aggressive lymphoma
Abstract
Background: Treatment of central nervous system relapse in adult acute lymphoblastic leukemia is a challenge and outcome is poor. Liposomal cytarabine has a prolonged half-life and, given intrathecally, has produced high response rates in patients with central nervous system relapse of non-Hodgkin's lymphoma. The aim of this study was to evaluate the efficacy and tolerability of liposomal cytarabine in central nervous system relapse of acute lymphoblastic leukemia or Burkitt's lymphoma/leukemia.
Design and methods: Liposomal cytarabine (50 mg) was given intrathecally together with systemic or intrathecal dexamethasone once every 2 weeks in a phase II European trial. The primary end-point, cytological response in the cerebrospinal fluid after one or two cycles, was evaluated at the time of next treatment.
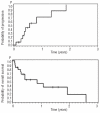
Results: Nineteen heavily pretreated patients (median age, 53 years; range 24-76 years) were evaluable: 14 with acute lymphoblastic leukemia and 5 with Burkitt's lymphoma/leukemia). Complete cytological remission as best response after two cycles of liposomal cytarabine was confirmed in 74% of the patients: 86% of those with acute lymphoblastic leukemia and 40% of those with Burkitt's lymphoma/leukemia). Nine of the 14 patients who achieved complete remission relapsed after a median of 7 months. The median overall survival was 11 months. Adverse events were observed in 89% of the patients (57% of cycles). Grade III-IV events with potential correlation to liposomal cytarabine occurred in 32% of the patients. The most frequent adverse event was headache. One patient developed severe neurological complications with loss of vision and a conus syndrome.
Conclusions: Overall, liposomal cytarabine showed excellent antileukemic activity. Toxicity was acceptable but appeared to increase with the number of cycles. Future evaluation in prophylaxis is of interest.
Trial registration: ClinicalTrials.gov NCT00199108.
Figures
References
-
- Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Sem Hematol. 2009;46(1):64–75. - PubMed
-
- Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–68. - PubMed
-
- Gökbuget N, Aguion-Freire E, Diedrich H, Digel W, Faak T, Kasper C, et al. Characteristics and outcome of CNS relapse in patients with acute lymphoblastic leukemia (ALL) Blood. 1999;94(10):1287a.
-
- Surapaneni UR, Cortes JE, Thomas D, O'Brien S, Giles FJ, Koller C, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia. Cancer. 2002;94 (3):773–9. - PubMed
-
- Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical